Considering Plant-Based Meat Substitutes and Cell-Based Meats: A Public Health and Food Systems Perspective
- 1Johns Hopkins Center for a Livable Future, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 2Department of Environmental Health & Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 3Department of Political Science, Johns Hopkins University, Baltimore, MD, United States
- 4Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 5Risk Sciences and Public Policy Institute, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
Over the past decade, there has been growing interest in the development and production of plant-based and cell-based alternatives to farmed meat. Although promoted for their capacity to avoid or reduce the environmental, animal welfare, and, in some cases, public health problems associated with farmed meat production and consumption, little research has critically evaluated the broader potential public health and food systems implications associated with meat alternatives. This review explores key public health, environmental, animal welfare, economic, and policy implications related to the production and consumption of plant-based meat substitutes and cell-based meats, and how they compare to those associated with farmed meat production. Based on the limited evidence to date, it is unknown whether replacing farmed meats with plant-based substitutes would offer comparable nutritional or chronic disease reduction benefits as replacing meats with whole legumes. Production of plant-based substitutes, however, may involve smaller environmental impacts compared to the production of farmed meats, though the relative impacts differ significantly depending on the type of products under comparison. Research to date suggests that many of the purported environmental and health benefits of cell-based meat are largely speculative. Demand for both plant-based substitutes and cell-based meats may significantly reduce dependence on livestock to be raised and slaughtered for meat production, although cell-based meats will require further technological developments to completely remove animal-based inputs. The broader socioeconomic and political implications of replacing farmed meat with meat alternatives merit further research. An additional factor to consider is that much of the existing research on plant-based substitutes and cell-based meats has been funded or commissioned by companies developing these products, or by other organizations promoting these products. This review has revealed a number of research gaps that merit further exploration, ideally with independently funded peer-reviewed studies, to further inform the conversation around the development and commercialization of plant-based substitutes and cell-based meats.
Introduction
Interest in plant-based substitutes and cell-based meats—collectively referred to as meat alternatives hereafter—has grown rapidly over the past decade. While some consumers choose to avoid meat from farmed animals (hereafter “farmed meat”) or animal foods altogether, a growing number of people are replacing a share of their meat intake with “plant-based substitutes” that seek to approximate the texture, flavor, and/or nutrient profiles of farmed meat using ingredients derived from pulses, grains, oils, and other plants and/or fungi. These products may soon be joined by “cell-based meats” (also referred to as “cultured meat,” “in-vitro meat,” “lab-grown meat,” “cellular meat,” “cultivated meat,” or “clean meat”) grown from animal stem cells using tissue engineering techniques, which currently remain for the most part in the prototype stage of development.
The global market for plant-based substitutes is projected to reach $85 billion (USD) by 2030, up from $4.6 billion (USD) in 2018 (Gordon et al., 2019). At the same time, while cell-based meat is not yet commercially available, research and development are proceeding rapidly. One think tank estimates that demand for beef and dairy products in the U.S. will shrink by 80–90% by 2035, driven largely by a projection that the cost of “modern protein foods” (including certain plant-based substitutes and cell-based meats) will be five times cheaper than existing animal proteins (Tubb and Seba, 2019). Although these estimates are speculative, and not necessarily supported by other industry experts, they emphasize the disruptive potential of meat alternatives on the animal agriculture sector.
Meat alternatives are often promoted as a means of mitigating the environmental, animal welfare, and, in some cases, public health problems associated with farmed meat production and consumption while appealing to mainstream consumers through existing supply chains. Growing scientific consensus has established that substantial shifts toward plant-forward diets, particularly in high meat-consuming countries, are essential for meeting climate change mitigation targets (Bajželj et al., 2014; Hedenus et al., 2014; Bryngelsson et al., 2016) and remaining within planetary boundaries (Willett et al., 2019). At the same time, there has been increased attention to the negative public health (Casey et al., 2015; Godfray et al., 2018) and animal welfare [Pew Commission on Industrial Animal Farm Production (PCIAFP), 2008] impacts of industrial food animal production, the prevailing model of meat production in the U.S. and increasingly in other parts of the world (Lam et al., 2019). A growing body of evidence has also associated red and processed meat consumption with certain chronic diseases and early mortality (Micha et al., 2012; Pan et al., 2012). Taken together, these concerns have driven efforts to reduce consumption of meat from farmed animals. Acknowledging that farmed meat production is not homogenous, in cases where the bulk of evidence is applicable only to meat from industrial food animal production, we use the term “conventional meat” to exclude more agroecological alternatives.
Seafood alternatives are also being developed to address concerns about the depletion of many of the world's wild fisheries [Food and Agriculture Organization of the United Nations (FAO), 2014] and the environmental impacts and constraints associated with many forms of farmed fish (i.e., aquaculture) production (Fry et al., 2016). There is almost no research examining the production or consumption of seafood alternatives, but we assume that many of the implications may be inferred from research on terrestrial meat alternatives since they are derived from similar ingredients. Thus, unless otherwise indicated, the terms “meat alternatives,” “plant-based substitutes,” and “cell-based meats” include seafood alternatives for simplicity of reading.
To date, few studies have critically evaluated the purported benefits of meat alternatives. To address this gap, this review explores the potential public health, environmental, animal welfare, economic, and policy implications associated with the production and consumption of plant-based substitutes and cell-based meats, and how they compare to those associated with farmed meat. Our findings are based on the best available evidence in the peer-reviewed academic literature, and in some cases, selected reports and other gray literature. We note limitations and research debates whenever possible.
The subsequent sections are laid out as follows: overview of concerns and considerations regarding farmed meat and seafood; discussion of the promises of meat and seafood alternatives; public health, environmental, animal welfare, economic, and policy implications associated with meat alternatives; conclusion; and suggested steps for further research. Appendix A (Supplementary Material) provides detailed methods of the literature search used for this review.
Background: Concerns and Considerations Regarding Farmed Meat and Seafood
Below we summarize some of the key public health and food systems concerns and considerations associated with farmed meat and seafood production and consumption in order to inform the evaluations of meat alternatives that purportedly attempt to mitigate some of these concerns in the subsequent sections. Livestock production systems have the potential for both positive (e.g., nutrient recycling) and negative (e.g., nutrient pollution) outcomes; the former include contributions of grazing systems to protein security and ecosystem services, which we discuss below, as well as to landscape aesthetic, gastronomic heritage, and other social and cultural factors (Ryschawy et al., 2019) that are beyond the scope of this review.
Public Health
Epidemiologic studies have linked Western dietary patterns that are high in the consumption of animal products, processed foods, refined sugars, and fats with escalating rates of chronic diseases. Red and processed meat consumption, in particular, has been associated with increased risks of heart disease and type 2 diabetes (Micha et al., 2012), stroke (Kaluza et al., 2012), certain cancers (particularly colorectal) (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2018), and all-cause mortality (Pan et al., 2012; Larsson and Orsini, 2014; Zheng et al., 2019). A nascent body of exploratory literature suggests that the consumption of certain compounds in animal foods (e.g., L-carnitine, found primarily in red meat) may promote the growth of intestinal microbiota that produce metabolites associated with an increased risk for cardiovascular disease and inflammatory bowel disease (Koeth et al., 2013, 2019).
In these studies, the term “red meat” includes beef, pork, lamb, and veal, and “processed meat” includes meats preserved using high levels of salt and/or chemical preservatives (e.g., bacon, hot dogs, sausage); these health risks are not necessarily documented for unprocessed versions of white meats such as chicken and turkey (Micha et al., 2012). While several studies have modeled potential population-level health benefits of reduced red and processed meat consumption (Smed et al., 2016; Springmann et al., 2016, 2018), it is important to recognize that animal-source foods, including meat, can be a valuable source of protein and bioavailable micronutrients, especially for young children and in the absence of accessible plant-based alternatives (Semba, 2016).
In contrast to the health concerns associated with red and processed meat consumption, regular consumption of seafood—particularly “oily” fish and certain mollusks rich in omega-3 fatty acids—has been associated with many health benefits, including a reduced risk of cardiovascular disease in adults and improved cognitive development during gestation and infancy (Mozaffarian and Rimm, 2006). That said, there is not enough seafood available globally for everyone to consume at the recommended levels to reap the noted health benefits, even accounting for the growth of aquaculture (Thurstan and Roberts, 2014).
While food safety concerns are not exclusively tied to animal foods, many of the bacterial pathogens responsible for foodborne illness—such as Salmonella, Escherichia coli, Campylobacter, and Listeria—live in the guts of animals. Pathogens of animal origin can enter the food supply via multiple pathways, such as if manure is transported via runoff onto nearby produce fields or contaminates water sources used for irrigation (Solomon et al., 2002; Erickson and Doyle, 2012). More directly, if animals' digestive tracts are accidentally severed during processing and slaughtering, the spilled contents may contaminate meat with the potential for widespread cross-contamination. These concerns are heightened by the potential presence of antibiotic-resistant pathogens on meat (Waters et al., 2011), a hazard linked to the misuse of antibiotics in industrial food animal production (Silbergeld et al., 2008; Haskell et al., 2018).
Beyond risks to consumers, workers in industrial food animal production operations may be exposed to zoonotic pathogens—including antibiotic-resistant strains—and wide a range of airborne hazards (Fitch et al., 2017); an estimated one in four workers in indoor confinement operations suffer from some form of respiratory illness (Donham et al., 2007). Aquaculture workers may similarly contend with bacterial, respiratory, injury and other occupational hazards (Myers, 2010). Although not exclusive to the farmed meat and seafood industries, animal slaughtering and meat processing workers are often required to perform strenuous labor for long hours under hazardous conditions, and face high rates of injury and illness (Fitch et al., 2017).
Neighbors living close to industrial food animal production operations face elevated risks of respiratory outcomes, stress, negative moods, and infection with zoonotic pathogens, including methicillin-resistant Staphylococcus aureus (Casey et al., 2015). More than just an unpleasant smell, strong odors from industrial operations can interfere with daily activities, social gatherings, and overall quality of life, and have been implicated in adverse physical and mental health outcomes (Horton et al., 2009; Wing et al., 2013). Communities may additionally face health risks associated with waterborne bacterial and chemical hazards originating from nearby operations (Burkholder et al., 2007).
Environmental
Livestock production accounts for an estimated 14.5 percent of global greenhouse gas (GHG) emissions from human activities (Gerber et al., 2013). Meat and dairy from ruminant animals (e.g., cattle, goats), farmed crustaceans (e.g., shrimp, prawns), and trawled lobster are particularly GHG-intensive (Clune et al., 2017; Poore and Nemecek, 2018; Kim et al., 2019). Some research suggests that under specific soil, climate, and animal density conditions, well-managed grazing livestock may sequester carbon, thus lowering the GHG footprints of ruminant products (Tichenor et al., 2017); however, other research contends that this effect is time-limited, reversible, and potentially outweighed by other GHGs generated by grazing systems (Garnett et al., 2017).
The estimated amount of land devoted to livestock production ranges from 2.5 (Mottet et al., 2017) to 3.7 billion ha (Foley et al., 2011)—roughly half to three-quarters of global agricultural land—while animal foods account for only 18% of calories and 25% of protein in the global food supply (Mottet et al., 2017). This is in part due to the amount of forage and feed required to produce an equivalent amount of calories and protein from meat as could be provided directly from plants grown for human consumption, with the caveat that animal proteins generally are more bioavailable to humans and have all essential amino acids in sufficient amounts (Cassidy et al., 2013). Beef is particularly land-intensive compared to other meats (Poore and Nemecek, 2018), in part because cattle have a slower reproductive cycle and are less efficient at converting feed to meat (Nijdam et al., 2012).
Despite the relatively large land footprint of farmed animals, there are two important and related considerations regarding the contributions of grazing ruminants to land use and protein security. First, in contrast to poultry, pork, and increasingly farmed fish (Fry et al., 2016)—which are fed crops grown on land that could otherwise be used to grow crops for direct human consumption—ruminant animals can graze on land that is unsuitable, e.g., too rocky or too hilly, for crop production. Of the 2.5 billion ha devoted to livestock production, 1.3 billion ha are non-arable grasslands (Mottet et al., 2017). Thus, reducing beef production and consumption would not necessarily free up a proportional amount of land to feed people or other livestock (Peters et al., 2016). Second, farmed animals, particularly grazing ruminants, can convert plants that are inedible to humans into human-edible proteins. Grassland-based systems in the United Kingdom, for example, were found to provide 1.1 kg protein from beef and 1.4 kg protein from milk per kg of human-edible plant protein from feed and forages. By contrast, poultry, pork, and grain-fed beef provided only 0.5, 0.4, and 0.3 kg protein, respectively, per kg human-edible plant protein (Wilkinson, 2011; Peyraud and Peeters, 2016). Grassland production systems thus present an opportunity to contribute to protein security; grain-fed systems, however, remain the predominant model of livestock production in industrialized countries. Within the US, for example, only 1% of the current beef supply comes from exclusively pasture-based systems, though the potential exists to produce up to 27–35% of the current beef supply using exclusively pasture (Hayek and Garrett, 2018). On average globally, ruminant meat currently relies on cropland to the same extent per unit of protein as pork and poultry (Herrero et al., 2015).
With a few exceptions, more inputs into feed production (e.g., water, pesticides, fertilizers) are needed to produce the same amounts of calories and protein in meat compared to plant foods intended for direct human consumption (Marlow et al., 2009). Livestock production as an industry also contributes more to biodiversity loss (Machovina et al., 2015) and disruptions in nutrient cycles that exacerbate groundwater pollution and eutrophication (Bouwman et al., 2013) than the production of crops for human consumption. Eutrophication occurs when excess nutrient levels (primarily nitrogen and phosphorus) cause toxic algae blooms that deplete oxygen levels in the water and kill fish, plants, and other aquatic life. Resource inputs and the associated impacts may be reduced with agroecological approaches such as integrated crop-livestock and/or multi-species farming, and well-managed pasture-based livestock production systems in general; these approaches can also provide other ecological services including reducing dependence on synthetic fertilizers through nutrient recycling, fostering soil health, and sustaining biodiversity of grassland ecosystems (Janzen, 2011; Röös et al., 2017; Martin et al., 2020).
Animal Welfare
Over 9.5 billion terrestrial animals were slaughtered for meat in the US in 2017 [U.S. Department of Agriculture (USDA), 2019], with global estimates at around 75 billion terrestrial animals [Food and Agriculture Organization of the United Nations (FAO), 2020]. Global meat production (in tonnage) has increased over 4.5-fold from 1961 to 2018, nearly twice the rate of population growth [Food and Agriculture Organization of the United Nations (FAO), 2020]. Industrial food animal production is designed to produce abundant amounts of meat, eggs, or milk rapidly and at minimal cost. Most operations raise animals in crowded facilities, often in confined crates or cages, without outdoor access or the ability to exhibit their natural behaviors [Pew Commission on Industrial Animal Farm Production (PCIAFP), 2008]. Animals in many cases are subject to painful bodily alterations (e.g., debeaking, dehorning, castration), often without pain relief [Pew Commission on Industrial Animal Farm Production (PCIAFP), 2008]. Animal welfare problems may exist on small-scale, organic, or pasture-based farms, too; such operations do not necessarily have higher animal welfare standards [Pew Commission on Industrial Animal Farm Production (PCIAFP), 2008].
Economic
In much of the industrialized world, traditionally diversified farms have been replaced over the past century with operations that specialize in producing specific crops or animals at a large scale, buoyed by mechanization, standardization, and increased off-farm inputs (e.g., pesticides, pharmaceuticals) (Ikerd, 2008). Large multi-national corporations have consolidated small businesses and other corporations to control multiple stages along the food supply chain, including in the meat processing and marketing industry (Weis, 2013; Howard, 2016). Such systems are credited with improving efficiency, reducing costs, and lowering consumer prices, but are also implicated in the decline in workers' wages (Oxfam America, 2015); the loss of farmers' and public autonomy over the food system (Ikerd, 2008; IPES-Food, 2017); and the deterioration of rural communities and economies (Lobao, 1990; Stofferahn, 2006), including local property values (Keeney, 2008).
The Promises of Meat Alternatives
A variety of alternatives exist to approximate or even replicate certain aspects of meat's texture, flavor, and/or nutrient profile. These range from natural foods that resemble certain characteristics—not necessarily nutritional—of meat (e.g., pulses, mushrooms, jackfruit), to products that are not designed to mimic meat but can be used in similar ways (e.g., tofu, tempeh, seitan, bean burgers), to more processed products that are designed to imitate the experience of eating certain meat products (e.g., meat-like burgers, hot dogs, fish filets) (Lagally et al., 2017).
Products in the last category have been gaining particular momentum over the past decade, with new technological advances aimed at replicating selected characteristics of meat down to the molecular level. Several products are designed to be “viscerally equivalent” to farmed meats in order to appeal to those who enjoy meat (Stephens et al., 2018). Most of these plant-based substitutes use soy, wheat, or pea protein isolates or concentrates as their primary protein source, though products derived from fungi (i.e., mycoprotein) and lupin beans also exist. Examples of common plant-based substitute brands and products include Gardein Meatless Meatballs, Morningstar Farms Original Chik Patties, Beyond Meat's Beyond Burger and Impossible Foods' Impossible Burger (see Table S3). A rapidly growing number of companies are also aspiring to produce cell-based meats that are not only viscerally equivalent but also “biologically equivalent” to farmed meat through cultivation of animal cells (Stephens et al., 2018). The technological feasibility of replicating the exact structure, texture, color, flavor, and nutritional composition of farmed meat, however, remains in question. Replicating these characteristics for fresh, unprocessed meat would require several particularly complex technical feats, including simulating the role of blood in delivering oxygen and nutrients throughout thicker pieces of tissue, as well as co-culturing fat, muscle, and connective tissues (Fraeye et al., 2020).
Meat alternatives are promoted for their environmental, animal welfare, and in some cases, public health benefits. “Eat Meat. Save Earth,” is the mission proclaimed on Impossible Foods' website (Impossible Foods, 2020), accompanied with statistics comparing the land, water and GHG emissions associated with an Impossible Burger and a conventional beef burger. Popular press echoes these messages about how “Fake Meat Will Save Us” (Egan, 2019). As one journalist states: “Farmfree food will allow us to hand back vast areas of land and sea to nature, permitting rewilding and carbon drawdown on a massive scale. It means an end to the exploitation of animals, an end to most deforestation, a massive reduction in the use of pesticides and fertilizer, the end of trawlers and longliners” (Monbiot, 2020). Cell-based meat is also purported to be “healthier, safer, and disease-free” compared to farmed meat (Arshad et al., 2017). Notably, these claims are most often compared to beef, which generally has the largest environmental impacts among animal products.
The extent to which meat alternatives achieve these purported benefits depends in part on several factors, including the specific ingredients or inputs used to produce them (Figures 1, 2), the extent to which consumers accept and incorporate these products into their diets, and which farmed meats they are replacing (e.g., beef vs. poultry, conventional meat vs. meat from agroecological production systems), if any. Thus, in the following sections, we compare the impacts of meat alternatives to a variety of farmed meats. Although several literature reviews have examined trends in consumer perceptions about and theoretical willingness to try meat alternatives (Hartmann and Siegrist, 2017; Bryant and Barnett, 2018; Weinrich, 2019), the studies underlying these reviews may be outdated given the influx of new plant-based substitutes into the market and demonstrated consumer acceptance in the past few years [International Food Information Council (IFIC), 2020; McCarthy and DeKoster, 2020]. As cell-based meats enter the marketplace, consumer perceptions and acceptance may also change. We also recognize that potential public health, environmental, and animal welfare benefits associated with meat alternatives would only occur if demand for those products offsets a share of farmed meat production, rather than simply adding to the combined total production of farmed meat and meat alternatives (Stephens et al., 2018). Given the importance of consumption patterns on the potential benefits associated with meat alternatives, we call for additional research in Appendix B (Supplementary Material) to better understand how consumers are incorporating these products into their diets.
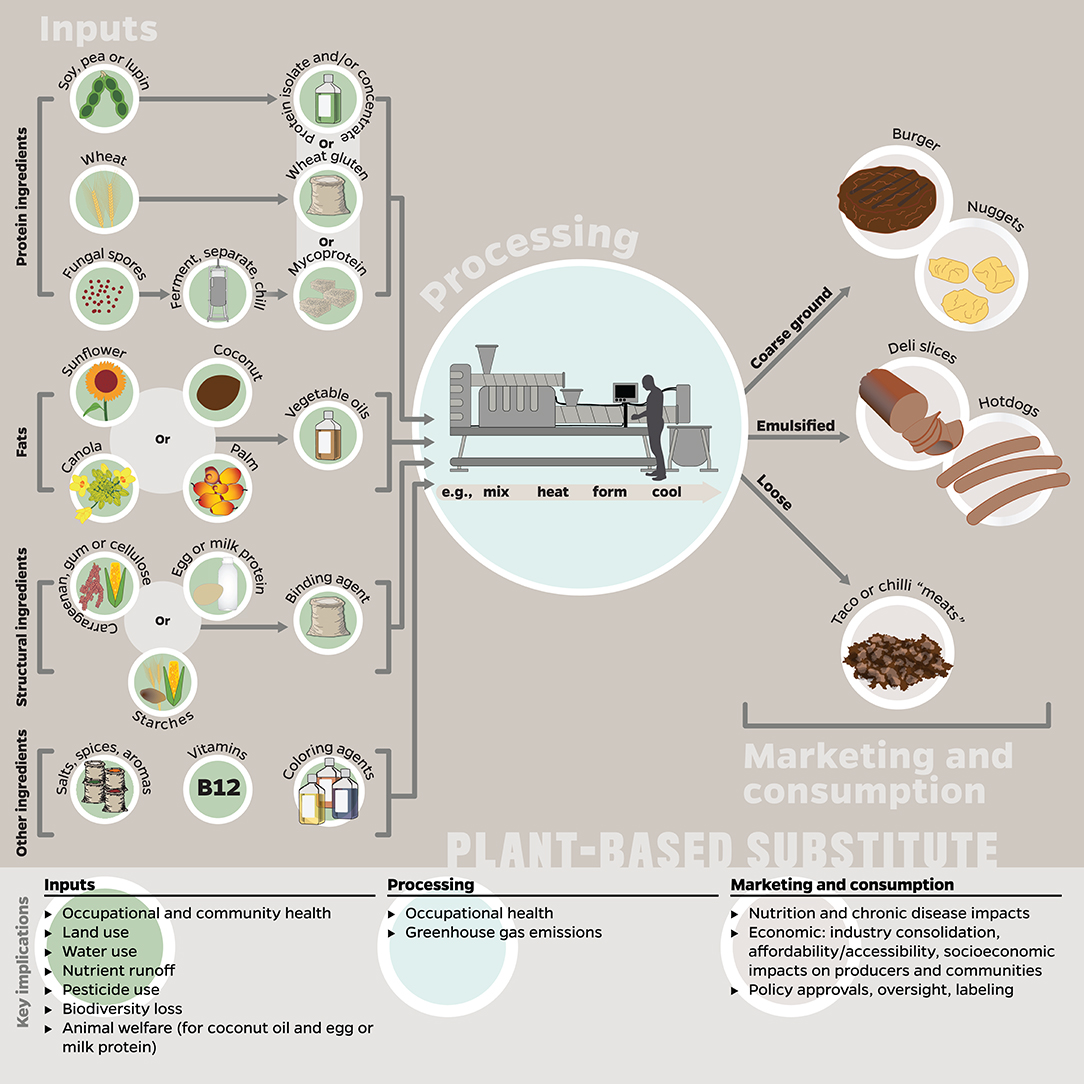
Figure 1. Potential inputs, processes, and final product(s) to be marketed and consumed as plant-based substitutes, and how these stages correspond to key implications explored in this paper. Many of the implications listed here are applicable to multiple stages, e.g., GHG emissions occur in the production of inputs, processing, and retail/consumer stages; however, we listed each implication only with the stage to which it is most relevant or has the greatest impact. The inputs represent a compilation of ingredients included in plant-based substitutes; most products do not contain all of these ingredients at once. This figure was designed by the authors using information reported in Joshi and Kumar (2015), Bohrer (2019), and Kyriakopoulou et al. (2019).
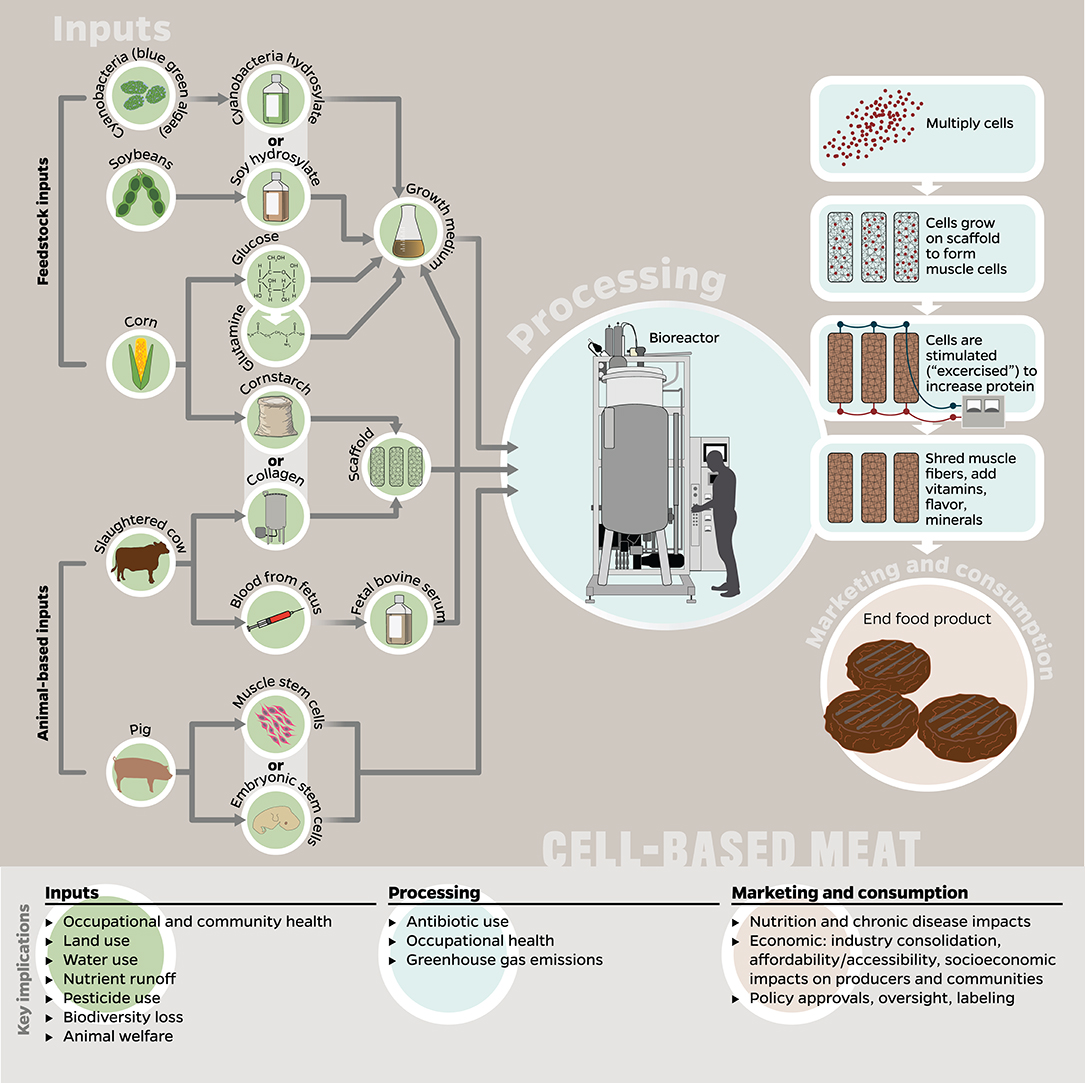
Figure 2. The potential inputs, processes, and final product(s) to be marketed and consumed as cell-based meats, and how these stages correspond to key implications explored in this paper. Many of the implications listed here are applicable to multiple stages, e.g., GHG emissions occur in the production of inputs, processing, and retail/consumer stages; however, we listed each implication only with the stage to which it is most relevant or has the greatest potential impact. Since no products are currently available on the market, the figure was designed by the authors using hypothetical inputs proposed by Tuomisto and Teixeira de Mattos (2011) and Mattick et al. (2015b) as well as currently required animal-based inputs (e.g., fetal bovine serum, collagen-based scaffolds) (Stephens et al., 2018; Thorrez and Vandenburgh, 2019), although we recognize the goal to eliminate the latter eventually.
It is worth mentioning that since cell-based meat has not yet been commercialized, existing research about its production is based on a few anticipatory life cycle assessments (LCAs) which assumed hypothetical inputs, production processes, and technological advances (Tuomisto and Teixeira de Mattos, 2011; Tuomisto et al., 20141; Mattick et al., 2015b). Some researchers have noted that several assumptions and simplifications made in these LCAs are not supported by existing scientific evidence and should be interpreted carefully (Lynch and Pierrehumbert, 2019; Thorrez and Vandenburgh, 2019). For instance, the presented LCAs covered in this review assumed that the cell-based meat would be grown without fetal bovine serum, a reality that remains one of the industry's biggest (see Inputs). Nevertheless, we include those studies' results, since it is the most detailed information about the potential inputs and implications of cell-based meat production. Given the limitations of existing research, it is of critical importance that ongoing, independent, and comprehensive multi-product environmental analyses are conducted as the technologies and commercial operations for meat alternatives develop and scale (Mattick et al., 2015a).
Many plant-based seafood substitutes use soy, wheat, or pea protein isolates as their primary protein source (Table S4) and are comparable to plant-based terrestrial meat substitutes. Some products on the market are not designed to mimic seafood exactly but can be used in similar ways (e.g., products made from carrots, eggplant, or tomatoes); these are not examined in this review. Additionally, while the term “seafood” includes sea vegetables (e.g., seaweed, algae)—some of which may have high concentrations of protein and micronutrients (Fleurence et al., 2012)—their impacts are not assessed here. Cell-based seafood products are also in development, though the regulatory pathways and markets will likely be different than those of cell-based terrestrial meats.
Lastly, while this review primarily compares meat alternatives to the farmed meats for which they are intended to substitute, meeting dietary protein needs does not necessarily require consumption of either group of products. Producing and consuming other protein-rich foods, such as minimally processed legumes (including soybeans, lentils, beans, and peas) and insects, should be considered as part of the path forward for sustainable food systems.
Public Health Implications
Here we review the array of public health implications associated with meat alternatives, exploring the nutrition, chronic disease, and food safety implications associated with consuming them and the occupational and community health impacts associated with their production.
Nutrition and Chronic Disease
In general, many plant-based substitutes contain comparable amounts of calories, protein, and iron as the meats they are intended to replace (Bohrer, 2019). As ultra-processed foods, plant-based substitutes have relatively high amounts of sodium compared to unprocessed meats and may contain ingredients and additives including flavoring, coloring, and binding agents (Bohrer, 2019; Curtain and Grafenauer, 2019). Tables S3, S4 highlight key ingredients in plant-based burgers and seafood substitutes, respectively, from some top retail brands in the U.S. market. Several products contain coconut oil; among those in Table S3 that contain coconut oil, their saturated fat levels are lower than that of beef, but comparable to or higher than those of poultry and pork. These ingredients and additives are not necessarily beneficial or harmful to human health from a nutrition perspective. For example, despite consumer perceptions of coconut oil as a health-promoting food, evidence of its health benefits is lacking, though more robust research is merited (Lockyer and Stanner, 2016). At the same time, although consumer desires for “clean labels” have prompted concerns about the use of certain binding agents and gums in plant-based substitutes, research has shown methylcellulose and guar gum to have similar cholesterol and glucose-lowering effects as other dietary fibers (Mudgil et al., 2014; Kuczora, 2015; Bohrer, 2019). See also Food Safety for potential food safety concerns associated with additives.
Although they may contain similar macronutrient profiles, replacing meat with a plant-based substitute does not necessarily reflect a healthy dietary pattern (Hu et al., 2019). A plant-based burger or hot dog may be served with a refined bun, few vegetables, and nutrient-poor sides such as fries or chips. Similarly, seafood substitutes could theoretically be fortified with omega-3 fatty acids, but it is unknown whether doing so would provide comparable health benefits to eating whole unprocessed fish. Furthermore, consumption of ultra-processed foods is associated with greater caloric intake and weight gain (Hall et al., 2019) and a range of adverse long-term health outcomes (Lawrence and Baker, 2019). Further research is needed to determine whether plant-based substitutes are replacing processed or unprocessed foods in people's diets, and if they can ultimately lead to healthier dietary patterns.
By contrast, dietary patterns rich in whole plant-based foods such as legumes, whole grains, vegetables and nuts have been associated with a reduced risk for chronic diseases and adverse health outcomes (Nelson et al., 2016). While plant-based substitutes are primarily derived from legumes, it is unknown whether substitutes derived from plant protein isolates offer similar nutritional benefits or chronic disease reductions as whole legumes (Hu et al., 2019). Soy protein isolates (containing >90% soy protein) or concentrates (70–90% soy protein), for instance, are primary ingredients in many plant-based substitutes (Malav et al., 2015). Whole soy foods (e.g., edamame, tempeh) and minimally processed soy foods (e.g., full-fat tofu and soymilk) are complex foods rich in protein (including all the essential amino acids), omega-3 fatty acids, and many biologically active components (most notably isoflavones) (Omoni and Aluko, 2005). Soy food and/or protein consumption, either in comparison to animal protein intake or in the form of supplementation, has been associated with improved blood lipid levels (Anderson et al., 1995; Reynolds et al., 2006), moderately improved measures of bone health (Zhang et al., 2005; Bawa, 2010), reduced menopausal symptoms (Franco et al., 2016), reduced risk of type 2 diabetes (Tang et al., 2020), and modestly decreased breast cancer risk (Fritz et al., 2013). While some benefits associated with soy consumption were studied using soy protein isolates or extracts, these lack some of the beneficial nutritional components found in whole soybeans (Messina and Messina, 2010), partly as a result of the manufacturing processes used to extract protein (Erdman, 2000). For that reason, consuming whole soy foods is generally recommended over consuming isolated soy components (Michelfelder, 2009; Messina and Messina, 2010). Further research comparing the health effects of whole soy foods to plant-based substitutes made with isolated soy proteins—and likewise, whole peas to plant-based substitutes made with isolated pea proteins—is merited.
Plant-based diets have also been associated with more diverse gut microbiomes than omnivorous diets, though this may largely be due to compounds and characteristics of whole plants consumed in plant-based diets (Tomova et al., 2019). It is unclear if or how meat alternatives would impact the gut microbiome and associated health outcomes (Hu et al., 2019).
An additional consideration specific to Impossible Foods' plant-based substitutes relates to the heme iron (in the form of soy leghemoglobin) content, the ingredient that imparts the product's “meaty” flavor and aroma. High levels of heme iron intake from red and processed meat consumption have been associated with elevated risk for type 2 diabetes (Bao et al., 2012), cardiovascular disease (Fang et al., 2015), colorectal cancer (Bastide et al., 2011; Fonseca-Nunes et al., 2014; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2018) and lung cancer (Tasevska et al., 2009; Fonseca-Nunes et al., 2014). Impossible Foods has reported that the heme in its beef substitute is comparable in amount and, once cooked and digested, identical molecularly to that found in farmed beef [GRAS Notice (GRN) No. 737, 2017], suggesting that consumption of this plant-based substitute may be associated with similar chronic disease risks as red and processed meat consumption. That said, consumption of heme iron—the most easily absorbed form of iron—is associated with a reduced risk for iron deficiency, a prevalent nutritional concern for women of childbearing age and pregnant and lactating women globally (Zimmermann and Hurrell, 2007).
As cell-based meats are not yet commercially available, there is little information available about their nutritional content. On the one hand, while developers aspire to replicate the nutrition profile of farmed meat, many unanswered questions remain about the technological feasibility of achieving this in vitro, particularly with regard to the quality and composition of proteins, amino acids, vitamins, minerals, fatty acids, and compounds such as taurine and creatine (Fraeye et al., 2020). On the other hand, some of these attributes could be leveraged to enhance the nutritional value over that of farmed meat; proponents have claimed that the quantity and type of fat could be controlled, and that other functional ingredients, such as vitamin C or omega 3 fatty acids, could be added to the growth medium (Post, 2012; Bhat et al., 2019).
Food Safety
Most plant-based substitutes contain at least one major food allergen among their ingredients, with wheat and soy being the most common [Food Drug Administration (FDA), 2004]. Individuals allergic to peanuts and soy may also experience reactions to pea and lupin protein, though this is rare (Lavine and Ben-Shoshan, 2019). Allergic and gastrointestinal reactions to mycoprotein-based plant-based substitutes (e.g., Quorn) have also been reported; though rare, the incidence of adverse reactions to mycoprotein in the general population is debated (Jacobson and DePorter, 2018; Finnigan et al., 2019). Individuals with intolerances to certain food additives and gums must also be careful given their prevalence in plant-based substitutes.
Carrageenan, for example, is a structural ingredient derived from seaweed that is commonly used in plant-based substitutes and other processed foods for purposes of thickening, gelling, or stabilizing. The safety of carrageenan has long been debated, with attention being focused on its potential to elicit gastrointestinal inflammation, alterations to intestinal microflora, and other related outcomes such as irritable bowel syndrome and colon cancer (Bixler, 2017; David et al., 2018). Additionally, because carrageenan is grown in seawater, it has the potential to accumulate significant concentrations of heavy metals (Almela et al., 2002; Besada et al., 2009), though no research has characterized exposures to arsenic, cadmium, lead, and mercury that result from consumption of carrageenan-containing foods.
Some concerns have also been raised about the safety of new additives present in some plant-based substitutes, such as mycoprotein used in Quorn products and soy leghemoglobin used in Impossible Foods products. See Policy Implications for a discussion of the approval processes and regulatory debates.
Some propose that if cell-based meat were produced under sterile conditions, it could reduce the incidence of foodborne illness (Bhat and Bhat, 2011). By not involving the processing of whole animal carcasses, cell-based meats would likely reduce the potential for contamination that exists in farmed meat handling and processing, such as Escherichia coli contamination from contact with digestive organs and feces. However, fully sterile conditions would be near impossible to achieve and thus antibiotics would likely be required as inputs for the tissue culture medium in order to inhibit the growth of bacterial pathogens (Stephens et al., 2018; Thorrez and Vandenburgh, 2019). The exact nature of antibiotic use in this context is not yet known, though the quantities and regularity of use would likely be lower than in industrial livestock operations. Transmission of zoonotic diseases may decline if cell-based meat production reduced human-livestock interactions (Bhat and Bhat, 2011; Arshad et al., 2017), though more research on this potential is merited.
Occupational Health
There is little known about occupational exposure risks incurred by workers in plant-based substitute manufacturing, though they are likely less hazardous than those faced by farmed meat processing workers (see Public Health). One consumer advocacy group has raised concerns about the use of hexane in processing soy protein isolates used in plant-based substitutes (Vallaeys et al., 2010). It may also be used to process pea protein isolates, though less information on this is available (Tömösközi et al., 2001; Holt, 2018). Hexane is a neurotoxic and highly explosive solvent and also a hazardous air pollutant [Environmental Protection Agency (EPA), 2000]. To our knowledge, no specific information is available on the amount of hexane used in the production of soy and pea protein isolates, and on the extent of measures to protect workers, prevent environmental releases, and monitor exposures.
Given the level of uncertainty regarding the specific laboratory processes and regulatory landscape that will emerge for cell-based meat production (see Regulatory Oversight of Cell-Based Meat), occupational health and safety implications for cell-based meat workers remain unclear.
Community Health
Both plant-based substitutes and (hypothetically) cell-based meats rely on crops that are already significant parts of the agricultural system, including soybeans, wheat, and corn (Figures 1, 2). In addition to contributing to nutrient runoff that can contaminate local groundwater sources, the production of these crops often involves pesticides associated with long-term chronic health problems for people who work on and live near farms (Harrison, 2011). Concerns have also been raised that the use of low levels of some herbicides in soybean production, including dicamba; 2,4-D; and glyphosate may induce multiple-antibiotic resistance in pathogens, compromising the effectiveness of life-saving medicines (Kurenbach et al., 2015). Additionally, the heavy use of agricultural fungicides, such as in the production of peas and soybeans, has been implicated in the rise of resistance to anti-fungal medicines, which has particularly serious consequences for immunosuppressed individuals (Revie et al., 2018). All of this said, because it takes more soy used as animal feed to produce one conventional meat burger compared to the amount of soy used as an ingredient in a plant-based burger, conventional meat often requires more pesticides to produce than plant-based substitutes (see Pesticide Use). The relative risks for community health associated with plant-based substitutes and cell-based meats compared to conventional meats, and also more agroecologically produced meats, should be more thoroughly evaluated.
It also remains to be seen whether potential antibiotic use and waste management practices associated with cell-based meat production will impact people who work on or live near production facilities, as they do with industrial food animal production.
Environmental Implications
The environmental impacts of meat alternatives depend largely on two stages of production: (1) the agricultural production of inputs and (2) the processing of inputs into final products. For plant-based substitutes, these inputs include primary ingredients, e.g., soybeans, wheat, peas, fungi, and lupins (Figure 1). For cell-based meat, inputs provide energy or nutrients to the cell medium; the specific inputs that will be used for commercial production are unknown largely because of their proprietary nature. Hypothetical studies of cell-based meat development have modeled production using cyanobacteria (i.e., blue-green algae) or compounds derived from soybeans and corn as inputs, neither of which are necessarily viable (Thorrez and Vandenburgh, 2019) but provide a basis for initial analysis (Figure 2). The following section will review the GHG, land, water, pesticide use, eutrophication, and biodiversity implications associated with the production of meat alternatives compared to farmed meat production.
Greenhouse Gas Emissions
Based on our review of the literature (Figure 3; see Supplementary Data for details), the median GHG footprint of plant-based substitutes was 34, 43, 63, 72, 87, and 93% smaller than those of farmed fish, poultry meat, pig meat, farmed crustaceans, beef from dairy herds, and beef from beef herds, respectively, per 100 grams protein. Among the animal foods considered in our review, only wild tuna and insects were less GHG-intensive than plant-based substitutes. Plant-based substitutes were 1.6, 4.6, and 7.0 times more GHG-intensive than the less-processed plant proteins in this review, i.e., tofu, pulses (excluding peas), and peas, respectively. Only one study has quantified any environmental impacts associated with plant-based seafood substitutes (Quorn Foods, 2019); the GHG footprints were comparable to those of plant-based terrestrial meat substitutes made from similar ingredients, so we included those figures in the aggregate data for plant-based substitutes.
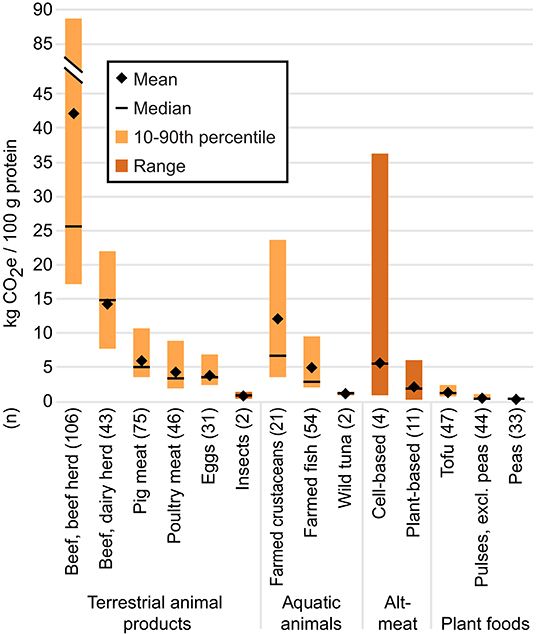
Figure 3. Cradle-to-processing gate GHG footprints (wherever possible) per 100 g protein. For cell-based meat, plant-based substitutes, insects, and wild tuna, the mean and median were calculated using the mean value from each individual study (n) to avoid over-representing results from studies that included more products than other studies. The minimum and maximum values of the range were derived from individual product footprints. For data sources and individual product footprints, see Supplementary Data. Data for all other foods are from Poore and Nemecek (2018); for these n represents the number of observations.
The hypothetical GHG footprint of cell-based meat varied significantly more than that of plant-based substitutes, from 0.9 to 36.3 kg CO2e/100 g protein (median: 5.6 kg CO2e/100 g protein). This variation was due to different assumptions embedded in the projections, such as the cell-based meat facility's size and the potential density and proliferation rates of cells (Mattick et al., 2015b). The median GHG footprint per 100 grams protein of cell-based meat was 17, 62, and 78% lower than those of farmed crustaceans, beef from dairy herds, and beef from beef herds, respectively, but 1.1 to 6.1 times higher than those of other animal products and 4.8, 13.4, and 20.6 times higher than those of tofu, pulses, and peas, respectively. The hypothetical GHG emissions associated with cell-based seafood products have not been explored, but would likely be similar to projections for terrestrial cell-based meat. The industrial energy requirements for cell-based meat were also higher than poultry meat in Mattick et al. (2015a) and poultry meat, pig meat, and beef in Tuomisto et al. (2014).
Given that a large proportion of the GHG footprint of plant-based substitutes and cell-based meat comes from the energy required to manufacture the products, these footprints could theoretically decrease if the energy grid were decarbonized. By contrast, significant reductions in the GHG-intensity of livestock production seem unlikely (Goldstein et al., 2017), with the caveat that emerging technologies to reduce methane from enteric fermentation may address a share of beef's emissions (Maia et al., 2016; Vyas et al., 2018).
Comparing the climate impacts of plant-based substitutes, cell-based meats, farmed meats, and seafood is complicated by varying atmospheric lifespans and global warming potentials among different GHGs. For example, methane remains in the atmosphere for a shorter period but has a more potent warming effect than carbon dioxide. Life cycle assessments generally standardize the warming potential of different GHGs (including carbon dioxide, methane, and nitrous oxide) in terms of carbon dioxide equivalents (CO2e), usually over a 100-year period. This metric, however, obscures the fact that a significant proportion of the GHG footprint of farmed beef is comprised of methane from enteric fermentation and manure decomposition, whereas the GHG footprints of meat alternatives are largely comprised of CO2 from electricity use, resulting in a more persistent but less intensive warming effect (Lynch and Pierrehumbert, 2019). The use of CO2e has raised debates in the academic and policy world, as the choice of metric and timeframe under consideration could result in very different policy priorities for reducing GHG emissions (Garnett, 2011; Allen, 2015). Over a 100-year time frame, for example, the GHG footprint of cell-based meat was found to be between 51 and 97% smaller compared to conventional beef produced in the Midwest U.S., whereas it was between 92% smaller to 9% larger using a 500-year time frame (Lynch and Pierrehumbert, 2019). The long atmospheric lifespan of carbon dioxide highlights the urgency of decarbonizing the energy grid, whether for cell-based meat production or any other energy-intensive activity.
Land Use
The median land use footprint of plant-based substitutes was 41, 77, 82, 89, and 98% smaller than that of farmed fish, poultry meat, pig meat, beef from dairy herds, and beef from beef herds, respectively, per 100 grams protein (Figure 4; see Supplementary Data). Thus replacing a share of farmed meat in the diet with plant-based substitutes could theoretically free up cropland to feed more people or provide other ecological services such as reforestation for carbon sequestration (Albanito et al., 2016) or the preservation of pasture-based livestock production systems that provide biodiversity benefits in certain landscapes (Röös et al., 2017). The median land use footprint of plant-based substitutes was 32, 52, and 75% smaller than that of tofu, peas and other pulses, respectively. These comparisons are skewed, however, by the fact that the values for less-processed plant proteins reflect global averages that include data from low-yielding countries (Poore and Nemecek, 2018), whereas the LCAs for plant-based substitutes likely assumed ingredients were sourced from more efficient production systems in industrialized countries.
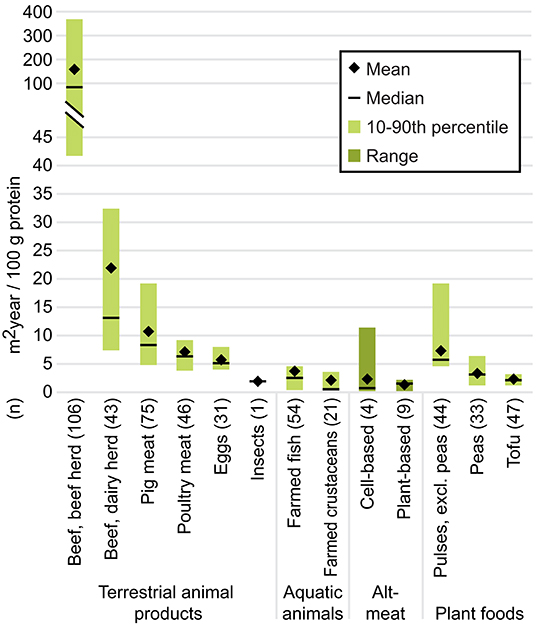
Figure 4. Land use per 100 g protein. For cell-based meat, plant-based substitutes, and insects, the mean and median were calculated using the mean value from each individual study (n) to avoid over-representing results from studies that included more products than other studies. The minimum and maximum values of the range were derived from individual product footprints. For data sources and individual product footprints, see Supplementary Data. Data for all other foods are from Poore and Nemecek (2018); for these n represents the number of observations.
The estimated land required to produce the ingredients for cell-based meat varied widely (0.1–11.5 m2 per year per 100 g protein; median: 0.6), depending largely on the choice of feedstock and inputs for the cell cultivation. Models of cell-based meat production using cyanobacteria (i.e., blue-green algae) hydrolysate (Tuomisto and Teixeira de Mattos, 2011; Tuomisto et al., 2014; Smetana et al., 2015) had the smallest land use requirements. Comparisons which modeled conventional livestock feeds (Alexander et al., 2017) or soy and corn-derived inputs (Mattick et al., 2015b) as the nutrients for the cell culture medium found that the land use requirements for cell-based meat were comparable to those of poultry when comparing based on protein content.
The land-sparing possibilities associated with meat alternatives would not necessarily occur with shifts away from farmed meat production. If farmed meat consumption were only reduced in industrialized countries, exports of feed crops could simply increase—though this could reduce demand on land clearing for agricultural use in other environmentally sensitive regions such as South America, where deforestation is a leading driver of climate change and biodiversity loss (Tilman et al., 2001; Machovina et al., 2015). If environmental land-sparing options were desired, it would be essential to have adequate policies preventing newly available land from being developed or used for other industrial purposes. It is also worth considering how significant changes in land use could impact rural communities where agriculture is often the economic driver (see Socio-Economic Implications).
Water Use
Fewer studies have quantified the amount of blue water (i.e., freshwater from ground or surface sources) consumed to produce meat alternatives. Based on our review of the available literature (Figure 5), per 100 grams protein, the median blue water footprint of plant-based substitutes was 21 and 42% smaller than those of pulses and soy; 76, 77, and 89% smaller than those of farmed poultry meat, bovine meat, and pig meat; and two orders of magnitude smaller than those of aquatic animals raised in ponds, e.g., farmed shrimp and tilapia. The values for pulses and soy were likely larger than those of plant-based substitutes in part because the former reflect global averages that include data from low-yielding countries (Kim et al., 2019), whereas the LCAs for plant-based substitutes likely assumed ingredients were sourced from more efficient production systems in industrialized countries. By contrast, the median blue water footprint of cell-based meat was larger than those of all other foods considered in our review except for those of farmed pig meat and pond-raised aquatic animals. See Supplementary Materials and Data for details.
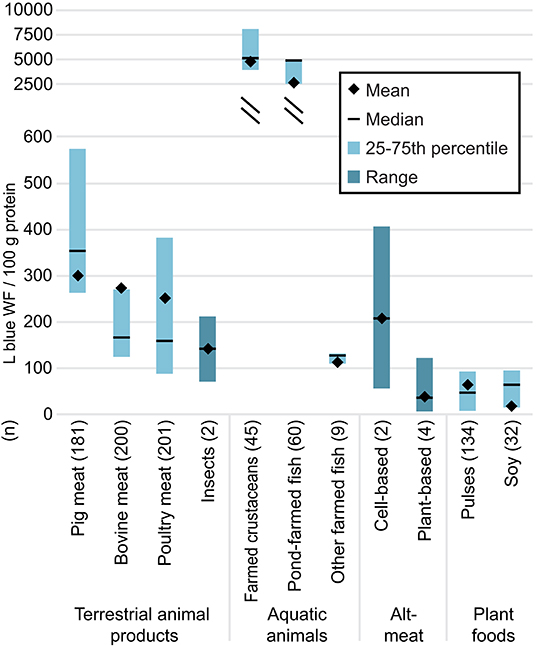
Figure 5. Blue water footprints per 100 g protein. For cell-based meat, plant-based substitutes, and insects, the mean and median were calculated using the mean value from each individual study (n) to avoid over-representing results from studies that included more products than other studies. The minimum and maximum values of the range were derived from individual product footprints. For data sources and individual product footprints, see Supplementary Data. Data for all other foods came from Kim et al. (2019); for these n represents the number of data points included.
Eutrophication
As highlighted in Table S3, many popular plant-based substitutes are derived from legumes, which in addition to their food value, are noted for their ability to improve soil fertility through fixing atmospheric nitrogen into a form that is usable by plants (Voisin et al., 2014). Incorporating legumes into crop rotations can diversify farmers' production systems and sources of income and reduce their dependency on synthetic nitrogen fertilizer (Voisin et al., 2014).
As with fertilized fields, nitrogen can leach from legume-based cropping systems into surface or ground water, which can contribute to eutrophication. Limited data exists on how much plant-based substitutes exacerbate eutrophication, but existing research suggests they provide significant benefits over conventional meats. One study found that the average freshwater eutrophication potential of plant-based substitutes was an order of magnitude smaller than that of conventional pork sausage patties, and two orders of magnitude smaller than those of beef and chicken patties (Fresán et al., 2019). Another study found that conventional pork production resulted in six times greater eutrophication potential and required 3.4 times more fertilizer per unit of protein compared to a pea-based plant-based substitute (Zhu and van Ierland, 2004). These findings are comparable to those of other studies that have found that growing pulses releases 85–94% less reactive nitrogen per unit of protein than producing seafood or conventional meat (Leach et al., 2016).
One study that modeled the hypothetical eutrophication potential of cell-based meat, based on inputs of soy hydrosylate and glucose and glutamine (both derived from corn), found it comparable to, or slightly lower than that of, conventional poultry production (Mattick et al., 2015b). This is expected given the inputs for the modeled cell culture were similar to those in poultry feed (e.g., corn, soy), and likely used in similar quantities based on the fact that poultry and cell-based meat production required roughly the same amount of land (Mattick et al., 2015b). For cell-based meat production systems that grow cyanobacteria as the primary input instead of corn or soy, nitrogen-fixing species of cyanobacteria could be selected to reduce the use of synthetic nitrogen fertilizer (Tuomisto and Teixeira de Mattos, 2011).
Pesticide Use
Limited research has explored the pesticide use associated with the production of meat alternatives. One study found that conventional pork production involved 1.6 times more pesticide use per unit of protein compared to the production of a pea-based plant-based substitute (Zhu and van Ierland, 2004). Another study found that conventional meat protein (an average of different animals) required six times more biocides (pesticides and disinfectants) to produce than the same amount of a soy-based plant-based substitute (Reijnders and Soret, 2003).
Conventionally grown soybeans, a common ingredient in plant-based substitutes as well as conventional animal feed, are among the most common crops genetically modified to be tolerant to herbicides such as glyphosate (i.e., “Roundup”); 2,4-D; and dicamba [U.S. Department of Agriculture Economic Research Service (USDA, ERS), 2019; U.S. Department of Agriculture (USDA), 2019]. Soybeans were the leading driver behind the growth in herbicide use in the U.S. from 1996 to 2011 and have contributed to the rise of herbicide-resistant “superweeds” (Benbrook, 2012). While the carcinogenicity of glyphosate to humans has been intensely debated (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2015; Williams et al., 2016), growing resistance has trapped farmers in a costly treadmill requiring them to apply more and multiple herbicides to control weeds (Benbrook, 2012).
The pesticide use involved in cell-based meat production depends largely on the inputs used in the culture medium. If soy and corn-based inputs were used as inputs, as modeled by Mattick et al. (2015b), it could be assumed that pesticide use in cell-based meat production would be comparable to that associated with conventional poultry production (since they required comparable amounts of land).
Biodiversity and Ecosystem Function
Producing legumes—the primary protein ingredient in most plant-based substitutes—can improve soil biodiversity and above-ground vegetative and invertebrate biodiversity, although the extent depends on management practices including tilling, chemical pest control, and fertilizer inputs (Williams et al., 2014). Soil biodiversity in turn promotes resistance and resilience against disturbance and stress, improves water and nutrient use efficiencies in crop production, and suppresses soil-borne disease (Brussaard et al., 2007).
Declining biodiversity of agricultural systems is also a concern for long-term food security and resilience, threatened in part by monoculture production systems and genetic uniformity in crop varieties and livestock breeds in conventional livestock production (Thrupp, 2000; Jackson et al., 2007). To the extent to which meat alternatives integrate ingredients other than soybeans and wheat (which are among the most produced crops worldwide, for both human foods and livestock feed), such as peas and lupins from which several plant-based substitutes are now derived, this could help diversify diets and foster agrobiodiversity.
Many plant-based substitutes include coconut or palm oil among their ingredients. Both of these plant-based lipids are grown in tropical regions rich in biodiversity, which is threatened by deforestation and anthropogenic forest disturbance (Barlow et al., 2016). Oil palm plantations have been a significant driver of deforestation and the associated biodiversity loss in Southeast Asia and South America (Vijay et al., 2016). While coconut plantations have not been implicated in significant demand-driven deforestation thus far, a massive scaling up of the plant-based substitute industry could pose biodiversity and sustainability concerns (Goldstein et al., 2017). That said, these concerns attributed to plant-based substitutes would also need to be evaluated in light of existing deforestation for pasture and feed crop production associated with conventional meat production (Goldstein et al., 2017).
Animal Welfare Implications
Meat alternatives, if widely adopted as a replacement for farmed meat, may greatly reduce dependence on livestock to be raised and slaughtered for meat production. That said, several technological challenges remain before animals can be completely removed from the supply chain of cell-based meat, including the source of the animal cell line and inputs used.
Source of Animal Cells
The first challenge relates to the source of the animal cell line. Cell-based meat production can occur in two ways, one of which requires only one animal and the other which requires a continuous stream of animals. In the first example, unfertilized eggs are obtained from a female animal and then fertilized by sperm in a petri dish (similar to in vitro fertilization) (Welin, 2013). If treated correctly, the embryonic stem cell line can be used indefinitely (Specht et al., 2018). While recent research suggests that these pluripotent cells could be manipulated into muscle fibers, it is a new technology and meat derived from them would require significant long-term safety testing, would have to be labeled as a genetically modified organism (i.e., GMO), and could undergo genetic mutations that might pose safety concerns or logistical challenges (Bhat et al., 2019; Thorrez and Vandenburgh, 2019).
Given these factors, the other source of animal cells—obtaining adult muscle stem cells from a biopsy of a living or dead animal—is currently the industry standard (Welin, 2013). Adult muscle stem cells can only replicate about 50–60 times before they reach their capacity to multiply and would need to be replaced (Kadim et al., 2015). A biopsy would also be required each time a new line of meat cells is produced (e.g., for each product a company develops). Additionally, while in theory substantially higher amounts of meat could be obtained per animal compared to animal slaughter for farmed meat (Stephens et al., 2018), the number or even magnitude of animals that would be implicated in each scenario has not, to our knowledge, been estimated in the literature. Furthermore, some have claimed that cell-based meat production could support the propagation of traditional native livestock breeds for cell harvesting in slaughter-free herds (Stephens et al., 2018), though it will depend upon the choices of the companies that commercialize cell-based meat. Comprehensive animal welfare assessments exploring the well-being of animals who are raised and undergo biopsies for the production of cell-based meat should be conducted.
No information is currently available on the extraction of cells from fish or shellfish to make cell-based seafood, such as whether they will come from wild or farmed fish, dead or alive. As these products develop, their animal welfare implications will have to be considered in the context of the debate over the extent to which fish or shellfish have the capacity to suffer and feel pain, and how animal welfare regulations established for terrestrial animals apply to commercial fishing and aquaculture operations (Huntingford et al., 2006; Browman et al., 2019).
Inputs
The second animal welfare challenge for cell-based meat concerns several inputs used that are still animal-derived due to technological or financial limitations. These include fetal bovine serum, scaffolds on which to grow the muscle tissue into thick pieces, and animal-derived hydrogels that are used to mimic natural tissue (Stephens et al., 2018). Fetal bovine serum (FBS), for instance, is a universal growth supplement for cell and tissue culture media extracted from the blood of a live cow fetus after the mother is slaughtered for meat processing (Gstraunthaler et al., 2013). While it is a byproduct of the meat industry (animals are not raised and slaughtered solely to produce FBS), its use means that cell-based meat production still hinges on farmed livestock production and raises several animal welfare concerns. The amount of serum obtained depends on the age of the fetus, but one 2002 study estimated that 800,000 liters of FBS were produced annually worldwide for use in culture media, corresponding to about 2 million bovine fetuses (Jochems et al., 2002). Demand for FBS has steadily increased worldwide, primarily due to use in drug and vaccine production and tissue engineering (Brunner et al., 2010). Serum-free growth media do exist and extensive research is dedicated to advance the field (van der Valk et al., 2018; Zhang et al., 2020). While they are currently prohibitively expensive (Specht et al., 2018; Thorrez and Vandenburgh, 2019), some prototypes have been shown to be able to effectively replace FBS, albeit less efficiently (Kolkmann et al., 2020). Until serum-free media becomes a viable option, more research into how many animals would be required to produce enough FBS for cell-based meat production is merited, though it will likely be far less than the volume of animals slaughtered for farmed meat production.
While most plant-based substitutes in theory do not contain animal products, the use of coconut oil in many plant-based substitutes raises animal welfare concerns. Many large coconut plantations in Thailand rely on monkeys, either stolen from the wild or bred on farm to harvest the coconuts. While there are some coconut oil producers that are “monkey free,” the continued employment of these highly intelligent animals in chained working conditions raises ethical dilemmas for the continued expansion of the coconut industry without specific standards on this issue (Barclay, 2015; Moyer, 2015). A small number of plant-based substitutes contain egg or milk protein, raising concerns about the welfare of laying hens and dairy cows, though the companies selling these products have recently been adding (e.g., Quorn Foods) and/or transitioning to 100% plant-based products (e.g., Morningstar Farms) (Blythman, 2018; Forgrieve, 2019).
Economic Implications
The following section explores how current trends in the development and production of meat alternatives may affect industry consolidation; consumer prices; and the economic well-being of small and mid-sized producers, rural communities, and less industrialized countries.
Industry Consolidation
Over the past decade, there has been significant investment in the research and development of meat alternatives (Mouat et al., 2019). Several of the leading meat processing and aggregation companies have announced they are developing their own plant-based substitutes (e.g., Tyson Foods, JBS, Nestle, Cargill, Hormel Foods, Perdue) or investing in existing ones; Tyson Foods, for example, was an early investor in Beyond Meat before starting its own product (Henderson, 2019). Other companies have been buying up existing plant-based substitute brands, e.g., Kellogg's owns Morningstar Farms, and Unilever acquired The Vegetarian Butcher (Lucas, 2019). Although cell-based meat production was initially developed by university-based researchers and in a few cases (e.g., Singapore Food Agency, 2020) driven by public-private investments, it is now primarily driven by venture-capital backed companies, some of which have received investment from large meat processing companies (Stephens et al., 2019).
The investment of agribusiness into the research and development of meat alternatives raises questions about who will benefit from the growth of this industry. Some have suggested that cell-based meat production could provide a new market opportunity for small businesses—akin to micro-brewery labs (van der Weele and Driessen, 2013; van der Weele and Tramper, 2014; Stephens et al., 2018). It is unclear, however, the extent to which smaller-scale “producers” will have access to government subsidies and grants or the technical information needed to produce cell-based meat, especially after it required so much capital for research and development (Stephens et al., 2018). The extent of intellectual property rights that will emerge around cell-based meat is also unclear, though analogous debates over seed patenting may be relevant (Barton and Berger, 2001). Some concerns have been raised that cell-based meats will allow multinational meat companies to assume more power in the food value chain (van der Weele and Driessen, 2013). Others have pointed out that since the vast majority of cell-based meat companies—as well as several plant-based substitute companies—are owned by agribusinesses or biotech startups headquartered in industrialized countries (Mouat et al., 2019), meat alternative industries could perpetuate economic and political power disparities between the Global North and South (Hocquette, 2016). It could be argued, however, that since attitudes toward and expectations about freshness in meats might be relaxed for meat alternatives (e.g., facilitating acceptance of frozen products), they could theoretically be produced further away from consumers than farmed meats and thus their production could potentially serve as an economic driver in less industrialized countries. It is also worth noting that proponents of meat alternatives would likely not disagree with such critiques, but argue that such products are not intended to address problems associated with agribusiness consolidation or globalization.
Socio-Economic Implications
If meat alternatives were to significantly replace farmed meat production, as some speculate (Tubb and Seba, 2019), it could have far-reaching socioeconomic effects on producers, workers, and rural communities. A rapid transformation of the agricultural marketplace from farmed to cell-based meat production—and, to a lesser extent, plant-based substitute production—could entail a significant overhaul in the labor workforce involved in protein production, from one largely based on farmers, farmworkers, meat processors, and veterinarians, to one based on chemists, cell biologists, engineers, and factory and warehouse workers (Mouat and Prince, 2018; Stephens et al., 2018). Although farmers and farmworkers would still need to produce raw ingredients or inputs for meat alternatives, a significant reduction in livestock production could contribute to massive layoffs and unemployment in the livestock farming and meat processing sectors. One report speculates that half of the 1.2 million jobs in U.S. beef and dairy production alone could be lost by 2030 and that farmland values could collapse by 40–80% due largely to its projection that “modern protein foods” will be five times cheaper than existing animal proteins (Tubb and Seba, 2019). It is unclear how many new jobs would be created by either plant-based substitute or cell-based meat industries. The status of trade agreements and tariffs, which remain a source of instability in both meat (Keefe, 2018) and crop (CoBANK Knowledge Exchange, 2019) markets, will also heavily influence whether livestock and feed crop farmers continue to produce their products and simply export more to other countries or industries, raise different animals, grow different crops, or sell their operations.
The implications of such drastic economic transitions should be further explored, especially for the well-being of farmers and farmworkers, who already experience poor mental health outcomes compared to other professions due to a variety of factors including financial stress, pesticide exposure, and climate variabilities (Daghagh et al., 2019). This is especially pertinent given recent media attention (the scientific literature has not caught up yet) to a looming economic and suicide crisis among American farmers considering the persistent agricultural recession, diminished farm income, rapidly increasing debts, and extreme flooding events (Harvie, 2017; Weingarten, 2018; Simpson, 2019). Moreover, if cell-based meat were produced in cities, it could also further perpetuate rural population loss and the associated disintegration of rural economies, which are largely dependent on agriculture (Tuomisto and Teixeira de Mattos, 2011; Johnson and Lichter, 2019; Pender et al., 2019). On the other hand, the potential for plant-based substitute and cell-based meat production to create new jobs in urban areas and in locations that do not have large agricultural workforces (e.g., Singapore) and in STEM fields in both the public and private sector is also worth analyzing. Although the rise of meat alternatives is but one of many factors affecting the agricultural marketplace, research and policies to support farmers in transitioning their farms to meet new market demands and to assist workers in relevant job retraining programs is merited.
Affordability/Accessibility
Price remains one of the most significant barriers for widespread adoption of plant-based substitutes and, especially, cell-based meats. While plant-based substitutes are becoming a competitive force in the marketplace, they comprise only a small overall market share and prices for most products are higher than those of farmed meats. Some proponents claim that plant-based substitutes will become cost-competitive with farmed meats as research and development costs are recouped, farmed meat processing companies enter the meat alternatives marketplace, manufacturing operations achieve economies of scale, and raw material varieties and prices are optimized (Specht, 2019). If plant-based substitutes do achieve price parity or eventually prove less expensive than farmed meats, it has been suggested that widespread market uptake could eventually make farmed meat a premium product, based on the assumption that plant-based substitutes would likely continue to replace lower quality products such as burgers (Bonny et al., 2015).
It also remains to be seen whether cell-based meat will be able to reach price parity with farmed meats. While the first cell-based meat burger for human consumption was produced in 2013 for an estimated $280,000 USD, one company (Biotech Foods) claims that they have now reduced the cost to 100 euros per kilogram, while another (Mosa Meat) projects it could be as low as $10 USD per burger by 2021 (González and Koltrowitz, 2019). The cost of animal-free growth medium is still around 50 times higher than what it would need to be cost-competitive with farmed meat, and that is only considering the cost of the growth medium (van der Weele and Tramper, 2014). If it does not achieve price parity, some have suggested cell-based meat could remain a niche product for wealthier consumers to avoid the guilt of consuming animal products (Cole and Morgan, 2013).
Depending on how the farmed meat production market is affected by the rise of meat alternatives, there could also be significant impacts on other industries that rely on the byproducts of farmed meat production, potentially affecting the cost of vaccines and other therapeutic substances as well as wool, cosmetics, and some pet food (Mattick et al., 2015a), unless these products are also replaced by cellular or acellular alternatives. That said, the costs of therapeutic and biomedical technologies relying on cell tissue engineering could also be decreased if affordable large-scale cell-based meat production were attained (Specht et al., 2018).
Policy Implications
The introduction of meat alternatives to the U.S. consumer market has fueled debates at the state and federal policy level. These debates center on food safety approvals, how to label plant-based substitute products, and in the case of cell-based meat, which agency will be responsible for overseeing production and marketing. Some of these debates stem from limitations in the existing regulatory framework around food production, inspection, and marketing, and are not unique to meat alternatives; however, critics have highlighted their importance in the context of these products.
Product Approvals
Concerns have been raised about how new ingredients in the food supply and food production processes are approved (IPES-Food, 2017), concerns which are relevant for the manufacturing of meat alternatives. Many food ingredient approval processes established by the US Food and Drug Administration (FDA) are voluntary and industry-led. For example, food companies can declare new substances they plan to use in food products to be generally recognized as safe (GRAS) based on their own risk assessments. Companies can voluntarily seek input from the FDA on their GRAS filings, but FDA notice or pre-market approval is not required (unlike the typical food additive safety review process) [Food and Drug Administration (FDA), 2016]. Some plant-based substitutes include novel food ingredients that have been introduced to the food supply through the GRAS process, including mycoprotein in Quorn products (Marlow Foods Ltd., 2001) and soy leghemoglobin in Impossible Foods products [GRAS Notice (GRN) No. 737, 2017]. In the case of soy leghemoglobin, a heme protein derived from genetically engineered yeast, Impossible Foods voluntarily submitted its GRAS determination to the FDA in 2017 and went through several rounds of questions and responses with the agency. During these exchanges, the agency determined that the reddish-brown color that soy leghemoglobin imparts in the company's uncooked beef substitutes qualified the ingredient as a color additive and thus required FDA approval before it could be sold to consumers in uncooked forms [Food and Drug Administration (FDA), 2019a]. In July 2019, the agency sent a notice saying it had no further questions about the ingredient's GRAS status and approved the company's petition to use soy leghemoglobin as a color additive [Food and Drug Administration (FDA), 2019b]. While some commentators have raised concerns about the process and decision (Storm, 2017; Lefferts, 2019), others find it sufficient to suggest that soy leghemoglobin is unlikely to pose a risk for consumers (Clinton, 2017; Johnson, 2017), though, to our knowledge, this has yet to be assessed in the academic literature. Consumer advocacy organizations are similarly concerned about the GRAS process in the context of cell-based meat (Hansen, 2018). A lawsuit was filed in 2017 challenging the GRAS self-certification process (Case 1:17-cv-03833, 2017).
Regulatory Oversight of Cell-Based Meat
The ontological challenges of deciding whether cell-based meat is considered “meat” or not have also posed practical questions in terms of which federal agency will be responsible for regulating production and inspection of cell-based meat in the U.S. After much deliberation, the FDA and USDA Food Safety and Inspection Service (USDA-FSIS) agreed to jointly regulate “human food products derived from the cultured cells of livestock and poultry” in March 2019 [Food and Drug Administration (FDA), 2019c]. The FDA will oversee the cell culturing stages of production from initial cell collection up to cell harvesting, at which point oversight will transition to the USDA-FSIS for meat production and labeling (Sancar, 2019). This division of responsibilities has been a longstanding challenge affecting other food products, but few as blatantly as cell-based meat. This division will not apply to cell-based seafood, which falls under the remit of FDA (Greene and Angadjivand, 2018).
While the joint agreement between FDA and USDA clarified many of the regulatory responsibilities between the agencies, some questions remain, stemming in part from the nature of how regulatory programs are established and funded. Congress enacts legislation that enables executive agencies to establish certain programs, creates budgets and appropriates federal funding for those programs (and the government generally), and provides oversight to ensure programs are running efficiently and funding is being spent appropriately. The development of regulations and programs within agencies through the enabling responsibility does not always align perfectly with Congressional appropriations, however, and conflicts may arise as a result. In the case of the joint agreement between FDA and USDA, the agreement does not empower either agency to spend additional resources on regulating cell-based animal products [Food and Drug Administration (FDA), 2019c]. Thus, the agencies' capacities to oversee these new industries may be limited by funding and personnel constraints, unless Congress authorizes additional funding for them.
Given the rapidly evolving technology involved in cell-based meat production, the regulatory frameworks surrounding it will likely change (Schneider, 2012; Stephens et al., 2018). As different types of cell-based meat products, production methods, and production facilities develop, they may require different regulatory approaches (Stephens et al., 2018). Other policy issues have yet to be addressed, including whether bioreactors will be considered agricultural facilities (which will affect zoning laws), whether waste will be regulated as an animal byproduct, the possibility for food fraud and mislabeling of cell-based and farmed meat products, and how to regulate cell-based meat produced using animal species not typically used for food (Stephens et al., 2018). Regulatory pathways and challenges will also differ between states and countries, depending in part on the strength and influence of the traditional agriculture lobby.
Labeling
There has been considerable debate around how meat alternatives should be labeled, with some livestock industry representatives concerned that consumers could be misled to think they are purchasing farmed meat. Federal legislation was introduced in October 2019 with the intention of limiting the widespread utilization of the term “meat” for products that are plant-based (U.S. House, 2019). The legislation would require labeling any product that does not contain “real” meat to bear the label “imitation” immediately before or after the name of the food, and enhance mislabeling enforcement provisions. This act comes after at least 25 states (O'Connor, 2019) have passed laws restricting use of the term “meat” or “beef” on plant-based substitutes or cell-based meat products. Given the negative connotations of the word “imitation,” in terms of both quality and flavor, these laws are likely intended to undermine the marketing of these products, rather than inform consumers who likely already know the nature of the meat alternatives they are buying. It is also worth mentioning that under FDA guidelines, “imitation” applies to any product that “resembles another food but is nutritionally inferior to that food,” such as imitation crab used in some sushi [Food and Drug Administration (FDA), 2019d]. Since some plant-based substitutes (and theoretically cell-based meat) contain comparable amounts of all essential nutrients of their farmed counterparts, it could be legally argued that these products are not “imitations.” This semantic and legal debate illustrates an interesting schism in the “meat industry”: the lobbyists advocating for these laws represent producers who are most likely to be affected by the rise of meat alternatives, rather than processing and manufacturing companies who stand to profit from the new products.
Conclusion
Plant-based substitutes and cell-based meats are gaining a foothold in global markets. This review of the evidence explores the extent to which the production and consumption of meat alternatives can mitigate some of the environmental, animal welfare, and public health problems associated with farmed meats, per how these products are often promoted. In doing so, we highlight the complexity of the issues at hand and the need for cautionary approaches to the rapid adoption of these products.
From an environmental perspective, plant-based substitutes can provide substantial benefits over farmed beef, to which they are most often compared by industry and media. Cell-based meat could provide benefits as well for most environmental concerns, with a few caveats: the GHG footprint, blue water footprint, and industrial energy use could be higher than those of farmed beef in some cases. Compared to farmed pork, poultry, eggs, and some types of seafood, the environmental benefits of meat alternatives are generally less pronounced (in the case of plant-based substitutes) or potentially non-existent (in the case of cell-based meat), a nuance that should be more transparent in discussions around meat alternatives.
From an animal welfare perspective, if meat alternatives replace even a small share of farmed meat production, this could substantially reduce the number of animals raised and killed for human protein consumption, demonstrating the ethical appeal of these products. Cell-based meat will, however, require further technological developments to remove all animal-based inputs including fetal bovine serum.
From a public health perspective, there has been limited research on nutrition, chronic disease, and food safety implications associated with consuming meat alternatives, and occupational and community health implications associated with their production. For example, it is unknown whether replacing farmed meat with plant-based substitutes would offer similar nutritional and health benefits as less-processed plant foods; the relative benefits would depend on the extent to which plant-based substitutes are replacing red and processed meat. Meanwhile, many of the purported health benefits of cell-based meat are largely speculative at this time, given the level of uncertainty around macro- and micronutrient content, the scope and nature of antibiotic use, and waste management practices.
The broader socioeconomic and political implications of widescale replacement of farmed meat with meat alternatives are also critical to consider, despite their frequent omission from most existing research. Meat alternatives are not intended to address concerns associated with industry consolidation, or the loss of farmers' and public autonomy over the food system, but as products to be offered within existing protein supply chains that appeal to those who enjoy meat but seek to reduce environmental, animal welfare, and public health harms. That said, these products illuminate important economic and political tensions between livestock producers and processing/marketing companies, between the workers who may benefit and those who may lose opportunities from their rise in popularity, and between consumers who may or may not be able to access them.
There is no silver bullet solution to addressing the myriad public health, environmental, and animal welfare challenges associated with protein consumption. While plant-based substitutes and cell-based meats may offer many benefits over some farmed meats, it is critical to remain cautious and nuanced in discussing their merits rather than assuming that they will solve our current challenges without any drawbacks. By the same token, these products should not be dismissed out of hand as fringe developments in the food system or as simply “imitations,” but should be approached with the same nuance as other foods. At the same time, the role of shifting toward more diverse, unprocessed whole foods (including pulses) while providing economic support for more agroecological producers—more than just substituting processed foods within otherwise unhealthy dietary patterns and inequitable supply chains—should not be overlooked. Mitigating the systemic problems of our food system will likely require the food processing and service industries, producers, and consumers to think beyond simply replacing one “meat” on a plate with another.
Future Research
This literature review has revealed a number of gaps in the research around plant-based substitutes and cell-based meats. Table 1 portrays the extent to which key public health, environmental, and animal welfare implications explored in this paper have been characterized in existing academic literature on meat alternatives. It is also worth mentioning that much of the existing environmental research on plant-based substitutes and cell-based meats has been funded or commissioned by companies developing these products, or by other non-profit organizations promoting these products (see Appendix in Supplementary Material Appendix A.2.1). In Appendix B (Supplementary Material), we highlight a list of specific research needs that should be further explored, ideally with independently funded peer-reviewed studies.
Table 1. Level of characterization of public health, environmental, and animal welfare implications in research to date on meat alternatives.
Author Contributions
SG and RS designed search protocol for academic databases. RS wrote the first draft of the manuscript. RS, SG, EB, and BK wrote sections of the manuscript. RS and BK compiled and standardized data for the figures and supplementary information provided. BK, JD, RN, and MB provided expertise on sections of the manuscript. KN provided guidance on all facets of and supervised the project. All authors reviewed and contributed to manuscript drafts and approved the submitted version.
Funding
This work was supported by the Santa Barbara Foundation (https://www.sbfoundation.org/). The funders had no role in preparing, reviewing, or editing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to express their gratitude to Grace Bachman, Katherine Sollee, Marissa Walsh, Sarah Wagner, and Jessica Ziesel for their research assistance; to Michael Milli for creating Figures 1, 2; and to Lori Rosman at the Johns Hopkins Welch Medical Library for her technical support and guidance on database reviews. The authors also thank Daphene Altema-Johnson, Dave Love, Robert Martin, Shawn McKenzie, Liz Nussbaumer, Becky Ramsing, Richard Semba, and Andrew Thorne-Lyman for providing comments on early drafts.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2020.00134/full#supplementary-material
Footnote
1. ^Tuomisto et al. (2014) is not a new LCA, but includes updates on some numbers reported in a previous study (Tuomisto and Teixeira de Mattos, 2011).
References
Albanito, F., Beringer, T., Corstanje, R., Poulter, B., Stephenson, A., Zawadzka, J., et al. (2016). Carbon implications of converting cropland to bioenergy crops or forest for climate mitigation: a global assessment. Gcb Bioenergy 8, 81–95. doi: 10.1111/gcbb.12242
Alexander, P., Brown, C., Arneth, A., Dias, C., Finnigan, J., Moran, D., et al. (2017). Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Global Food Security 15, 22–32. doi: 10.1016/j.gfs.2017.04.001
Allen, M. (2015). Short-Lived Promise? The Science and Policy of Cumulative and Short-Lived Climate Pollutants. Oxford: Oxford Martin School. Available online at: https://www.oxfordmartin.ox.ac.uk/downloads/briefings/Short_Lived_Promise.pdf
Almela, C., Algora, S., Benito, V., Clemente, M. J., Devesa, V., Súñer, M. A., et al. (2002). Heavy metal, total arsenic, and inorganic arsenic contents of algae food products. J. Agric. Food Chem. 50, 918–923. doi: 10.1021/jf0110250
Anderson, J. W., Johnstone, B. M., and Cook-Newell, M. E. (1995). Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 333, 276–282. doi: 10.1056/NEJM199508033330502
Arshad, M. S., Javed, M., Sohaib, M., Saeed, F., Imran, A., and Amjad, Z. (2017). Tissue engineering approaches to develop cultured meat from cells: a mini review. Cogent Food Agric. 3:1320814. doi: 10.1080/23311932.2017.1320814
Bajželj, B., Richards, K. S., Allwood, J. M., Smith, P., Dennis, J. S., Curmi, E., et al. (2014). Importance of food-demand management for climate mitigation. Nat. Clim. Chang. 4:924. doi: 10.1038/nclimate2353
Bao, W., Rong, Y., Rong, S., and Liu, L. (2012). Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 10:119. doi: 10.1186/1741-7015-10-119
Barclay, E. (2015). What's Funny About the Business of Monkeys Picking Coconuts? NPR The Salt. Available online at: https://www.npr.org/sections/thesalt/2015/10/19/448960760/monkeys-pick-coconuts-in-thailand-are-they-abused-or-working-animals
Barlow, J., Lennox, G. D., Ferreira, J., Berenguer, E., Lees, A. C., Mac Nally, R., et al. (2016). Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147. doi: 10.1038/nature18326
Barton, J. H., and Berger, P. (2001). Patenting agriculture. Issues Sci. Technol. 17, 43–50. Available online at: https://issues.org/barton/
Bastide, N. M., Pierre, F. H., and Corpet, D. E. (2011). Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. 4, 177–184. doi: 10.1158/1940-6207.CAPR-10-0113
Bawa, S. (2010). The significance of soy protein and soy bioactive compounds in the prophylaxis and treatment of osteoporosis. J. Osteoporosis 2010:891058. doi: 10.4061/2010/891058
Benbrook, C. M. (2012). Impacts of genetically engineered crops on pesticide use in the US–the first sixteen years. Environ. Sci. Europe 24:24. doi: 10.1186/2190-4715-24-24
Besada, V., Andrade, J. M., Schultze, F., and González, J. J. (2009). Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 75, 305–313. doi: 10.1016/j.jmarsys.2008.10.010
Bhat, Z. F., and Bhat, H. (2011). Animal-free meat biofabrication. Am. J. Food Technol. 6, 441–459. doi: 10.3923/ajft.2011.441.459
Bhat, Z. F., Morton, J. D., Mason, S. L., Bekhit, A. E.-D. A., and Bhat, H. F. (2019). Technological, regulatory, and ethical aspects of in vitro meat: a future slaughter-free harvest. Comprehens. Rev. Food Sci. Food Saf. 18, 1192–1208. doi: 10.1111/1541-4337.12473
Bixler, H. J. (2017). The carrageenan controversy. J. Appl. Phycol. 29, 2201–2207. doi: 10.1007/s10811-017-1132-4
Blythman, J. (2018). The Quorn revolution: the rise of ultra-processed fake meat. The Guardian. Available online at: https://www.theguardian.com/lifeandstyle/2018/feb/12/quorn-revolution-rise-ultra-processed-fake-meat
Bohrer, B. M. (2019). An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 8, 320–329. doi: 10.1016/j.fshw.2019.11.006
Bonny, S. P. F., Gardner, G. E., Pethick, D. W., and Hocquette, J.-F. (2015). What is artificial meat and what does it mean for the future of the meat industry? J. Integr. Agric. 14, 255–263. doi: 10.1016/S2095-3119(14)60888-1
Bouwman, L., Goldewijk, K. K., Van Der Hoek, K. W., Beusen, A. H., Van Vuuren, D. P., Willems, J., et al. (2013). Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. U. S. A. 110, 20882–20887. doi: 10.1073/pnas.1012878108
Browman, H. I., Cooke, S. J., Cowx, I. G., Derbyshire, S. W. G., Kasumyan, A., Key, B., et al. (2019). Welfare of aquatic animals: where things are, where they are going, and what it means for research, aquaculture, recreational angling, and commercial fishing. ICES J. Mar. Sci. 76, 82–92. doi: 10.1093/icesjms/fsy067
Brunner, D., Frank, J., Appl, H., Schöffl, H., Pfaller, W., and Gstraunthaler, G. (2010). The serum-free media interactive online database. ALTEX-Altern. Anim. Exp. 27, 53–62. doi: 10.14573/altex.2010.1.53
Brussaard, L., De Ruiter, P. C., and Brown, G. G. (2007). Soil biodiversity for agricultural sustainability. Agricul. Ecosyst. Environ. 121, 233–244. doi: 10.1016/j.agee.2006.12.013
Bryant, C., and Barnett, J. (2018). Consumer acceptance of cultured meat: a systematic review. Meat Sci. 143, 8–17. doi: 10.1016/j.meatsci.2018.04.008
Bryngelsson, D., Wirsenius, S., Hedenus, F., and Sonesson, U. (2016). How can the EU climate targets be met? A combined analysis of technological and demand-side changes in food and agriculture. Food Policy 59, 152–164. doi: 10.1016/j.foodpol.2015.12.012
Burkholder, J., Libra, B., Weyer, P., Heathcote, S., Kolpin, D., Thorne, P. S., et al. (2007). Impacts of waste from concentrated animal feeding operations on water quality. Environ. Health Perspect. 115, 308–312. doi: 10.1289/ehp.8839
Case 1:17-cv-03833 (2017). Plaintiffs Center for Food Safety, Breast Cancer Prevention Partners, Center for Science in the Public Interest, Environmental Defense Fund, and Environmental Working Group v. Tom Price, Secretary, Department Of Health And Human Services; Scott Gottlieb, Commissioner, United States Food And Drug Administration; And United States Food And Drug Administration (United States District Court Southern District of New York, May, 22). Available online at: http://www.centerforfoodsafety.org/files/1-complaint-2017-5-22_69110.pdf
Casey, J. A., Kim, B. F., Larsen, J., Price, L. B., and Nachman, K. E. (2015). Industrial food animal production and community health. Curr. Environ. Health Rep. 2, 259–271. doi: 10.1007/s40572-015-0061-0
Cassidy, E. S., West, P. C., Gerber, J. S., and Foley, J. A. (2013). Redefining agricultural yields: from tonnes to people nourished per hectare. Environ. Res. Lett. 8:034015. doi: 10.1088/1748-9326/8/3/034015
Clinton, P. (2017). The Impossible Burger Is Probably Safe. So Why Is Everyone Worked Up About “Heme”? The New Food Economy. Available online at: https://thecounter.org/plant-blood-soy-leghemoglobin-impossible-burger/
Clune, S., Crossin, E., and Verghese, K. (2017). Systematic review of greenhouse gas emissions for different fresh food categories. J. Clean. Prod. 140, 766–783. doi: 10.1016/j.jclepro.2016.04.082
CoBANK Knowledge Exchange (2019). The Year Ahead: Forces That Will Shape the U.S. Rural Economy in 2019. Available online at: https://www.cobank.com/-/media/files/ked/general/year-ahead-report-jan-2019.pdf?la=enandhash=C14485FF733F5E829AECCCF4849011536412768A
Cole, M., and Morgan, K. (2013). Engineering freedom? A critique of biotechnological routes to animal liberation. Configurations 21, 201–229. doi: 10.1353/con.2013.0015
Curtain, F., and Grafenauer, S. (2019). Plant-based meat substitutes in the flexitarian age: an audit of products on supermarket shelves. Nutrients 11:2603. doi: 10.3390/nu11112603
Daghagh, Y. S., Wheeler, S. A., and Zuo, A. (2019). Key risk factors affecting farmers' mental health: a systematic review. Int. J. Environ. Res. Public Health 16:4849. doi: 10.3390/ijerph16234849
David, S., Shani Levi, C., Fahoum, L., Ungar, Y., Meyron-Holtz, E. G., Shpigelman, A., et al. (2018). Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 9, 1344–1352. doi: 10.1039/C7FO01721A
Donham, K. J., Wing, S., Osterberg, D., Flora, J. L., Hodne, C., Thu, K. M., et al. (2007). Community health and socioeconomic issues surrounding concentrated animal feeding operations. Environ. Health Perspect. 115, 317–320. doi: 10.1289/ehp.8836
Egan, T. (2019, June 21). Fake meat will save us. The New York Times. Available online at: https://www.nytimes.com/2019/06/21/opinion/fake-meat-climate-change.html
Environmental Protection Agency (EPA) (2000). Hexane. Available online at: https://www.epa.gov/sites/production/files/2016-09/documents/hexane.pdf (accessed April 29, 2020).
Erdman, J. W. Jr. (2000). Soy protein and cardiovascular disease: a statement for healthcare professionals from the nutrition committee of the AHA. Circulation 102, 2555–2559. doi: 10.1161/01.CIR.102.20.2555
Erickson, M. C., and Doyle, M. P. (2012). “Plant food safety issues: linking production agriculture with One Health,” in Improving Food Safety Through a One Health Approach: Workshop Summary (Washington, DC: National Academies Press (US)).
Fang, X., An, P., Wang, H., Wang, X., Shen, X., Li, X., et al. (2015). Dietary intake of heme iron and risk of cardiovascular disease: a dose-response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 25, 24–35. doi: 10.1016/j.numecd.2014.09.002
Finnigan, T. J. A., Wall, B. T., Wilde, P. J., Stephens, F. B., Taylor, S. L., and Freedman, M. R. (2019). Mycoprotein: the future of nutritious nonmeat protein, a symposium review. Curr. Dev. Nutr. 3:nzz021. doi: 10.1093/cdn/nzz021
Fitch, C., Hricko, C., and Martin, R. (2017). Public Health, Immigration Reform and Food System Change. Baltimore, MD: Johns Hopkins Center for a Livable Future. Available online at: https://clf.jhsph.edu/publications/public-health-immigration-reform-and-food-system-change
Fleurence, J., Morançais, M., Dumay, J., Decottignies, P., Turpin, V., Munier, M., et al. (2012). What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 27, 57–61. doi: 10.1016/j.tifs.2012.03.004
Foley, J. A., Ramankutty, N., Brauman, K. A., Cassidy, E. S., Gerber, J. S., Johnston, M., et al. (2011). Solutions for a cultivated planet. Nature 478, 337–342. doi: 10.1038/nature10452
Fonseca-Nunes, A., Jakszyn, P., and Agudo, A. (2014). Iron and cancer risk—a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Prev. Biomark. 23, 12–31. doi: 10.1158/1055-9965.EPI-13-0733
Food Agriculture Organization of the United Nations (FAO) (2014). The State of World Fisheries and Aquaculture, Opportunities and Challenges. Rome. Available online at: http://www.fao.org/3/a-i3720e.pdf
Food Agriculture Organization of the United Nations (FAO) (2020). FAOSTAT/Data/Livestock Primary. Available online at: http://www.fao.org/faostat/en/#data/QL (accessed March 17, 2020).
Food Drug Administration (FDA) (2016). Substances Generally Recognized as Safe: A Rule by the Food and Drug Administration. Federal Register. Available online at: https://www.federalregister.gov/documents/2016/08/17/2016-19164/substances-generally-recognized-as-safe
Food Drug Administration (FDA) (2019a). Listing of Color Additives Exempt from Certification; Soy Leghemoglobin: A Rule by the Food and Drug Administration. Federal Register. Available online at: https://www.federalregister.gov/documents/2019/08/01/2019-16374/listing-of-color-additives-exempt-from-certification-soy-leghemoglobin
Food Drug Administration (FDA) (2019b). FDA in Brief: FDA Approves Soy Leghemoglobin as a Color Additive. Available online at: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-approves-soy-leghemoglobin-color-additive
Food Drug Administration (FDA) (2019c). Formal Agreement Between FDA and USDA regarding Oversight of Human Food Produced using Animal Cell Technology Derived from Cell Lines of USDA-Amenable Species. Available online at: https://www.fda.gov/food/domestic-interagency-agreements-food/formal-agreement-between-fda-and-usda-regarding-oversight-human-food-produced-using-animal-cell
Food Drug Administration (FDA) (2019d). Code of Federal Regulations Title 21, Volume 2. Part 101: Food Labeling. Available online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.3
Food Drug Administration (FDA) (2004). Food Allergen Labeling and Consumer Protection Act of (2004). (FALCPA). Available online at: https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa
Forgrieve, J. (2019). Morningstar Farms is Making the Transition From Vegetarian to Vegan. Forbes. Available online at: https://www.forbes.com/sites/janetforgrieve/2019/03/08/morningstar-farms-is-making-the-transition-from-vegetarian-to-vegan/#3344d53a44a0
Fraeye, I., Kratka, M., Vandenburgh, H., and Thorrez, L. (2020). Sensorial and nutritional aspects of cultured meat in comparison to traditional meat: much to be inferred. Front. Nutr. 7:35. doi: 10.3389/fnut.2020.00035
Franco, O. H., Chowdhury, R., Troup, J., Voortman, T., Kunutsor, S., Kavousi, M., et al. (2016). Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA 315, 2554–2563. doi: 10.1001/jama.2016.8012
Fresán, U., Marrin, D. L., Mejia, M. A., and Sabaté, J. (2019). Water footprint of plant-based substitutes: selected indicators according to life cycle assessment. Water 11:728. doi: 10.3390/w11040728
Fritz, H., Seely, D., Flower, G., Skidmore, B., Fernandes, R., Vadeboncoeur, S., et al. (2013). Soy, red clover, and isoflavones and breast cancer: a systematic review. PLoS ONE 8:e81968. doi: 10.1371/journal.pone.0081968
Fry, J. P., Love, D. C., MacDonald, G. K., West, P. C., Engstrom, P. M., Nachman, K. E., et al. (2016). Environmental health impacts of feeding crops to farmed fish. Environ. Int. 91, 201–214. doi: 10.1016/j.envint.2016.02.022
Garnett, T. (2011). Where are the best opportunities for reducing greenhouse gas emissions in the food system (including the food chain)? Food Policy 36, S23–S32. doi: 10.1016/j.foodpol.2010.10.010
Garnett, T., Godde, C., Muller, A., Röös, E., Smith, P., de Boer, I., et al. (2017). Grazed and Confused? Ruminating on Cattle, Grazing Systems, Methane, Nitrous Oxide, the Soil Carbon Sequestration Question – and What it All Means for Greenhouse Gas Emissions. Oxford: Food Climate Research Network. Available online at: https://www.fcrn.org.uk/sites/default/files/project-files/fcrn_gnc_report.pdf
Gerber, P. J., Steinfeld, H., Henderson, B., Mottet, A., Opio, C., Dijkman, J., et al. (2013). Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. Food and Agriculture Organization of the United Nations (FAO). Available online at: http://www.fao.org/3/a-i3437e.pdf
Godfray, H., Charles, J., Aveyard, P., Garnett, T., Hall, J. W., Key, T. J., et al. (2018). Meat consumption, health, and the environment. Science 361:eaam5324. doi: 10.1126/science.aam5324
Goldstein, B., Moses, R., Sammons, N., and Birkved, M. (2017). Potential to curb the environmental burdens of American beef consumption using a novel plant-based beef substitute. PLoS ONE 12:e0189029. doi: 10.1371/journal.pone.0189029
González, A., and Koltrowitz, S. (2019). The $280,000 Lab-Grown Burger Could Be a More Palatable $10 in Two Years. Reuters. Available online at: https://www.reuters.com/article/us-food-tech-labmeat/the-280000-lab-grown-burger-could-be-a-more-palatable-10-in-two-years-idUSKCN1U41W8
Gordon, W., Gantori, S., Gordon, J., Leemann, R., and Boer, R. (2019). The Food Revolution: The Future of Food and the Challenges We Face. USB. Available online at: https://www.ubs.com/global/en/wealth-management/chief-investment-office/investment-opportunities/sustainable-investing/2019/food-revolution.html
GRAS Notice (GRN) No. 737 (2017). GRAS Notification for Soy Leghemoglobin Protein Preparation Derived from Picha Pastoris. Submitted by Impossible Foods, Inc., Available online at: https://www.fda.gov/media/124351/download
Greene, J., and Angadjivand, S. (2018). Regulation of Cell-Cultured Meat. Congressional Research Service. IF10947. Available online at: https://crsreports.congress.gov/product/pdf/IF/IF10947/5
Gstraunthaler, G., Toni, L., and van der Valk, J. (2013). A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 65, 791–793. doi: 10.1007/s10616-013-9633-8
Hall, K. D., Ayuketah, A., Brychta, R., Cai, H., Cassimatis, T., Chen, K. Y., et al. (2019). Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 30, 67–77. doi: 10.1016/j.cmet.2019.05.008
Hansen, M. (2018). Foods Produced Using Animal Cell Culture Technology: Docket No. FDA-2018-N-2155. College Park, MD: FDA Public Meeting. Available online at: https://advocacy.consumerreports.org/wp-content/uploads/2018/07/CU-cmmts-to-FDA-on-lab-grown-meat-7.12.18-1.pdf
Harrison, J. L. (2011). Pesticide Drift and the Pursuit of Environmental Justice. Cambridge, MA: MIT Press.
Hartmann, C., and Siegrist, M. (2017). Consumer perception and behaviour regarding sustainable protein consumption: a systematic review. Trends Food Sci. Technol. 61, 11–25. doi: 10.1016/j.tifs.2016.12.006
Harvie, A. (2017). A Looming Crisis on American Farms. Farm Aid. Available online at: https://www.farmaid.org/blog/fact-sheet/looming-crisis-american-farms/
Haskell, K. J., Schriever, S. R., Fonoimoana, K. D., Haws, B., Hair, B. B., Wienclaw, T. M., et al. (2018). Antibiotic resistance is lower in Staphylococcus aureus isolated from antibiotic-free raw meat as compared to conventional raw meat. PLoS ONE 13:e0206712. doi: 10.1371/journal.pone.0206712
Hayek, M. N., and Garrett, R. D. (2018). Nationwide shift to grass-fed beef requires larger cattle population. Environ. Res. Lett. 13:084005. doi: 10.1088/1748-9326/aad401
Hedenus, F., Wirsenius, S., and Johansson, D. J. A. (2014). The importance of reduced meat and dairy consumption for meeting stringent climate change targets. Clim. Change 124, 79–91. doi: 10.1007/s10584-014-1104-5
Henderson, G. (2019). JBS to Launch Plant-Based Burger in Brazil. Drovers. Available online at: https://www.drovers.com/article/jbs-launch-plant-based-burger-brazil
Herrero, M., Wirsenius, S., Henderson, B., Rigolot, C., Thornton, P., Havlík, P., et al. (2015). Livestock and the environment: what have we learned in the past decade? Annu. Rev. Environ. Resour. 40, 177–202. doi: 10.1146/annurev-environ-031113-093503
Hocquette, J. F. (2016). Is in vitro meat the solution for the future? Meat Sci. 120, 167–176. doi: 10.1016/j.meatsci.2016.04.036
Holt, S. (2018). Your Pea Protein Primer. Civil Eats. Available online at: https://civileats.com/2018/04/02/your-pea-protein-primer/
Horton, R. A., Wing, S., Marshall, S. W., and Brownley, K. A. (2009). Malodor as a trigger of stress and negative mood in neighbors of industrial hog operations. Am. J. Public Health 99, S610–S615. doi: 10.2105/AJPH.2008.148924
Howard, P. H. (2016). Concentration and Power in the Food System: Who Controls what we Eat? Vol. 3. London; New York, NY: Bloomsbury Academic.
Hu, F. B., Otis, B. O., and McCarthy, G. (2019). Can plant-based meat alternatives be part of a healthy and sustainable diet? JAMA 322, 1547–1548. doi: 10.1001/jama.2019.13187
Huntingford, F. A., Adams, C., Braithwaite, V. A., Kadri, S., Pottinger, T. G., Sandøe, P., et al. (2006). Current issues in fish welfare. J. Fish Biol. 68, 332–372. doi: 10.1111/j.0022-1112.2006.001046.x
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2015). Some Organophosphate Insecticides and Herbicides. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 112. Lyon: International Agency for Research on Cancer (WHO). Available online at: https://monographs.iarc.fr/wp-content/uploads/2018/07/mono112.pdf
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2018). Red Meat and Processed Meat. IARC Monographs on the Evaluation or Carcinogenic Risks to Humans, Vol. 114. Lyon: International Agency for Research on Cancer. Available online at: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono114.pdf
Ikerd, J. E. (2008). Crisis and Opportunity: Sustainability in American Agriculture. Lincoln, NE: University of Nebraska Press.
Impossible Foods (2020). Mission. Available online at: https://impossiblefoods.com/mission/ (accessed March 30, 2020).
International Food Information Council (IFIC) (2020). A Consumer Survey on Plant Alternatives to Animal Meat. Available online at: https://foodinsight.org/wp-content/uploads/2020/01/IFIC-Plant-Alternative-to-Animal-Meat-Survey.pdf
IPES-Food (2017). Unravelling the Food–Health Nexus: Addressing Practices, Political Economy, and Power Relations to Build Healthier Food Systems. Brussels: IPES-Food. Available online at: http://www.ipes-food.org/_img/upload/files/Health_FullReport(1).pdf
Jackson, L. E., Pascual, U., and Hodgkin, T. (2007). Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 121, 196–210. doi: 10.1016/j.agee.2006.12.017
Jacobson, M. F., and DePorter, J. (2018). Self-reported adverse reactions associated with mycoprotein (quorn-brand) containing foods. Ann. Allergy Asthma Immunol. 120, 626–630. doi: 10.1016/j.anai.2018.03.020
Janzen, H. H. (2011). What place for livestock on a re-greening earth? Anim. Feed Sci. Technol. 166, 783–796. doi: 10.1016/j.anifeedsci.2011.04.055
Jochems, C. E. A., Van Der Valk, J. B. F., Stafleu, F. R., and Baumans, V. (2002). The use of fetal bovine serum: ethical or scientific problem? Altern. Lab. Anim. 30, 219–227. doi: 10.1177/026119290203000208
Johnson, K. M., and Lichter, D. T. (2019). Rural depopulation: growth and decline processes over the past century. Rural Sociol. 84, 3–27. doi: 10.1111/ruso.12266
Johnson, N. (2017). The Impossible Burger Wouldn't be Possible Without Genetic Engineering. Grist. Available online at: https://grist.org/article/the-impossible-burger-wouldnt-be-possible-without-genetic-engineering/
Joshi, V. K., and Kumar, S. (2015). Plant-based substituteues: plant based alternatives to meat products-a review. Int. J. Food Ferment. Technol. 5, 107–119. doi: 10.5958/2277-9396.2016.00001.5
Kadim, I. T., Mahgoub, O., Baqir, S., Faye, B., and Purchas, R. (2015). Cultured meat from muscle stem cells: a review of challenges and prospects. J. Integr. Agric. 14, 222–233. doi: 10.1016/S2095-3119(14)60881-9
Kaluza, J., Wolk, A., and Larsson, S. C. (2012). Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke 43, 2556–2560. doi: 10.1161/STROKEAHA.112.663286
Keefe, L. M. (2018). FakeMeat: how big a deal will animal plant-based substitutes ultimately be? Anim. Front. 8, 30–37. doi: 10.1093/af/vfy011
Keeney, R. (2008). Community Impacts of CAFOs: Property Values. Purdue Extension. Available online at: https://www.extension.purdue.edu/extmedia/ID/ID-363-W.pdf
Kim, B. F., Santo, R. E., Scatterday, A. P., Fry, J. P., Synk, C. M., Cebron, S. R., et al. (2019). Country-specific dietary shifts to mitigate climate and water crises. Glob. Environ. Change 62:101926. doi: 10.1016/j.gloenvcha.2019.05.010
Koeth, R. A., Lam-Galvez, B. R., Kirsop, J., Wang, Z., Levison, B. S., Gu, X., et al. (2019). L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Invest. 129, 373–387. doi: 10.1172/JCI94601
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., et al. (2013). Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19:576. doi: 10.1038/nm.3145
Kolkmann, A. M., Post, M. J., Rutjens, M. A. M., van Essen, A. L. M., and Moutsatsou, P. (2020). Cytotechnology 72, 111–120. doi: 10.1007/s10616-019-00361-y
Kuczora, S. (2015). “Authorised EU health claims for glucomannan, guar gum and hydroxypropyl methylcellulose,” in Foods, Nutrients and Food Ingredients with Authorised EU Health Claims, Vol. 2 (Duxford; Cambridge, MA; Kiddlington: Woodhead Publishing), 175–188. doi: 10.1016/B978-1-78242-382-9.00010-4
Kurenbach, B., Marjoshi, D., Amábile-Cuevas, C. F., Ferguson, G. C., Godsoe, W., Gibson, P., et al. (2015). Sublethal exposure to commercial formulations of the herbicides dicamba, 2, 4-dichlorophenoxyacetic acid, and glyphosate cause changes in antibiotic susceptibility in escherichia coli and Salmonella enterica serovar typhimurium. MBio 6:9. doi: 10.1128/mBio.00009-15
Kyriakopoulou, K., Dekkers, B., and van der Goot, A. J. (2019). “Plant-based substitutes,” in Sustainable Meat Production and Processing, ed C. M. Galanakis (Cambridge, MA: Academic Press), 103–126. doi: 10.1016/C2017-0-02230-9
Lagally, C., Clayton, E. R., and Specht, L. (2017). Plant-Based Meat Mind Maps: An Exploration of Options, Ideas, and Industry: The Good Food Institute. Available online at: https://www.gfi.org/files/PBMap.pdf
Lam, Y., Fry, J. P., and Nachman, K. E. (2019). Applying an environmental public health lens to the industrialization of food animal production in ten low-and middle-income countries. Global. Health 15:40. doi: 10.1186/s12992-019-0479-5
Larsson, S. C., and Orsini, N. (2014). Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am. J. Epidemiol. 179, 282–289. doi: 10.1093/aje/kwt261
Lavine, E., and Ben-Shoshan, M. (2019). Anaphylaxis to hidden pea protein: a Canadian pediatric case series. J. Allergy Clin. Immunol. Pract. 7, 2070–2071. doi: 10.1016/j.jaip.2019.02.010
Lawrence, M. A., and Baker, P. I. (2019). Ultra-processed food and adverse health outcomes. BMJ 365:l2289. doi: 10.1136/bmj.l2289
Leach, A. M., Emery, K. A., Gephart, J., Davis, K. F., Erisman, J. W., Leip, A., et al. (2016). Environmental impact food labels combining carbon, nitrogen, and water footprints. Food Policy 61, 213–223. doi: 10.1016/j.foodpol.2016.03.006
Lefferts, L. Y. (2019). ‘Barebones’ FDA Review of Impossible Burger's Soy Leghemoglobin Inadequate, Says CSPI. Center for Science in the Public Interest. Available online at: https://cspinet.org/news/barebones-fda-review-impossible-burger-soy-leghemoglobin-inadequate-20190903
Lobao, L. M. (1990). Locality and Inequality: Farm and Industry Structure and Socioeconomic Conditions. Albany, NY: SUNY Press.
Lockyer, S., and Stanner, S. (2016). Coconut oil–a nutty idea? Nutr. Bull. 41, 42–54. doi: 10.1111/nbu.12188
Lucas, A. (2019). Nestle Gears Up to Launch its Own Plant-Based Burger in the US. CNBC. Available online at: https://www.cnbc.com/2019/06/03/nestle-gears-up-to-launch-its-own-plant-based-burger-in-the-us.html
Lynch, J., and Pierrehumbert, R. (2019). Climate impacts of cultured meat and beef cattle. Front. Sustain. Food Syst. 3:5. doi: 10.3389/fsufs.2019.00005
Machovina, B., Feeley, K. J., and Ripple, W. J. (2015). Biodiversity conservation: the key is reducing meat consumption. Sci. Total Environ. 536, 419–431. doi: 10.1016/j.scitotenv.2015.07.022
Maia, M. R., Fonseca, A. J., Oliveira, H. M., Mendonça, C., and Cabrita, A. R. (2016). The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 6, 1–10. doi: 10.1038/srep32321
Malav, O. P., Talukder, S., Gokulakrishnan, P., and Chand, S. S. (2015). Plant-based substitute: a review. Crit. Rev. Food Sci. Nutr. 55, 1241–1245. doi: 10.1080/10408398.2012.689381
Marlow Foods Ltd. (2001). GRAS Determination for Mycoprotein. Available online at: https://wayback.archive-it.org/7993/20170607015621/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM266876.pdf (accessed December 20, 2019).
Marlow, H. J., Hayes, W. K., Soret, S., Carter, R. L., Schwab, E. R., and Sabaté, J. (2009). Diet and the environment: does what you eat matter? Am. J. Clin. Nutr. 89, 1699S−1703S. doi: 10.3945/ajcn.2009.26736Z
Martin, G., Barth, K., Benoit, M., Brock, C., Destruel, M., Dumont, B., et al. (2020). Potential of multi-species livestock farming to improve the sustainability of livestock farms: a review. Agric. Syst. 181:102821. doi: 10.1016/j.agsy.2020.102821
Mattick, C. S., Landis, A. E., and Allenby, B. R. (2015a). A case for systemic environmental analysis of cultured meat. J. Integr. Agric. 14, 249–254. doi: 10.1016/S2095-3119(14)60885-6
Mattick, C. S., Landis, A. E., Allenby, B. R., and Genovese, N. J. (2015b). Anticipatory life cycle analysis of in vitro biomass cultivation for cultured meat production in the United States. Environ. Sci. Technol. 49, 11941–11949. doi: 10.1021/acs.est.5b01614
McCarthy, J., and DeKoster, S. (2020). Four in 10 Americans Have Eaten Plant-Based Meats. Gallup. Available online at: https://news.gallup.com/poll/282989/four-americans-eaten-plant-based-meats.aspx
Messina, M., and Messina, V. (2010). The role of soy in vegetarian diets. Nutrients 2, 855–888. doi: 10.3390/nu2080855
Micha, R., Michas, G., and Mozaffarian, D. (2012). Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr. Atheroscler. Rep. 14, 515–524. doi: 10.1007/s11883-012-0282-8
Michelfelder, A. J. (2009). Soy: a complete source of protein. Am. Fam. Phys. 79, 43–47. Available online at: https://pubmed.ncbi.nlm.nih.gov/19145965/
Monbiot, G. (2020). Lab-grown food will soon destroy farming – and save the planet. The Guardian. Available online at: https://www.theguardian.com/commentisfree/2020/jan/08/lab-grown-food-destroy-farming-save-planet#maincontent
Mottet, A., de Haan, C., Falcucci, A., Tempio, G., Opio, C., and Gerber, P. (2017). Livestock: on our plates or eating at our table? A new analysis of the feed/food debate. Global Food Security 14, 1–8. doi: 10.1016/j.gfs.2017.01.001
Mouat, M. J., and Prince, R. (2018). Cultured meat and cowless milk: on making markets for animal-free food. J. Cult. Econ. 11, 315–329. doi: 10.1080/17530350.2018.1452277
Mouat, M. J., Prince, R., and Roche, M. M. (2019). Making value out of ethics: the emerging economic geography of lab-grown meat and other animal-free food products. Econ. Geogr. 95, 136–158. doi: 10.1080/00130095.2018.1508994
Moyer, J. W. (2015, Oct 20). They Murky ethics of making monkeys pick our coconuts. The Washington Post. Available online at: https://www.washingtonpost.com/news/morning-mix/wp/2015/10/20/the-murky-ethics-of-making-monkeys-pick-our-coconuts/
Mozaffarian, D., and Rimm, E. B. (2006). Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296, 1885–1899. doi: 10.1001/jama.296.15.1885
Mudgil, D., Barak, S., and Khatkar, B. S. (2014). Guar gum: processing, properties and food applications—a review. J. Food Sci. Technol. 51, 409–418. doi: 10.1007/s13197-011-0522-x
Myers, M. L. (2010). Review of occupational hazards associated with aquaculture. J. Agromed. 15, 412–426. doi: 10.1080/1059924X.2010.512854
Nelson, M. E., Hamm, M. W., Hu, F. B., Abrams, S. A., and Griffin, T. S. (2016). Alignment of healthy dietary patterns and environmental sustainability: a systematic review. Adv. Nutr. 7, 1005–1025. doi: 10.3945/an.116.012567
Nijdam, D., Rood, T., and Westhoek, H. (2012). The price of protein: review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 37, 760–770. doi: 10.1016/j.foodpol.2012.08.002
O'Connor, A. (2019, December 3). Fake Meat Vs. Real Meat. The New York Times. Available online at: https://www.nytimes.com/2019/12/03/well/eat/fake-meat-vs-real-meat.html
Omoni, A. O., and Aluko, R. E. (2005). Soybean foods and their benefits: potential mechanisms of action. Nutr. Rev. 63, 272–283. doi: 10.1111/j.1753-4887.2005.tb00141.x
Oxfam America (2015). Lives on the Line: The Human Cost of Cheap Chicken. Available online at: https://www.oxfamamerica.org/static/media/files/Lives_on_the_Line_Full_Report_Final.pdf
Pan, A., Sun, Q., Bernstein, A. M., Schulze, M. B., Manson, J. E., Stampfer, M. J., et al. (2012). Red meat consumption and mortality: results from 2 prospective cohort studies. Arch. Intern. Med. 172, 555–563. doi: 10.1001/archinternmed.2011.2287
Pender, J., Thomas, H., John, C., and Tracey, F. (2019). Rural America at a Glance, 2019. Edition. United States Department of Agriculture Economic Research Service. Available online at: https://www.ers.usda.gov/publications/pub-details/?pubid=95340
Peters, C. J., Picardy, J., Wilkins, J. L., Griffin, T. S., Fick, G. W., and Darrouzet-Nardi, A. F. (2016). Carrying capacity of US agricultural land: ten diet scenarios. Elem. Sci. Anthrop. 4:1. doi: 10.12952/journal.elementa.000116
Pew Commission on Industrial Animal Farm Production (PCIAFP) (2008). Animal Well-being. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health. Available online at: http://www.pcifapia.org/_images/212-7_PCIFAP_AmlWlBng_FINAL_REVISED_7-14-08.pdf
Peyraud, J.-L., and Peeters, A. (2016). The Role of Grassland Based Production System in the Protein Security. Grassland Science in Europe 21, Available online at: https://www.europeangrassland.org/fileadmin/documents/Infos/Printed_Matter/Proceedings/EGF2016.pdf
Poore, J., and Nemecek, T. (2018). Reducing food's environmental impacts through producers and consumers. Science 360, 987–992. doi: 10.1126/science.aaq0216
Post, M. J. (2012). Cultured meat from stem cells: challenges and prospects. Meat Sci. 92, 297–301. doi: 10.1016/j.meatsci.2012.04.008
Quorn Foods (2019). Quorn: Carbon Footprinting Emissions Report. Available online at: https://www.quorn.co.uk/files/content/Carbon_Footprint_Results_UK.pdf
Reijnders, L., and Soret, S. (2003). Quantification of the environmental impact of different dietary protein choices. Am. J. Clin. Nutr. 78, 664S−668S. doi: 10.1093/ajcn/78.3.664S
Revie, N. M., Iyer, K. R., Robbins, N., and Cowen, L. E. (2018). Antifungal drug resistance: evolution, mechanisms and impact. Curr. Opin. Microbiol. 45, 70–76. doi: 10.1016/j.mib.2018.02.005
Reynolds, K., Chin, A., Lees, K. A., Nguyen, A., Bujnowski, D., and He, J. (2006). A meta-analysis of the effect of soy protein supplementation on serum lipids. Am. J. Cardiol. 98, 633–640. doi: 10.1016/j.amjcard.2006.03.042
Röös, E., Bajželj, B., Smith, P., Patel, M., Little, D., and Garnett, T. (2017). Protein futures for western Europe: potential land use and climate impacts in 2050. Reg. Environ. Change 17, 367–377. doi: 10.1007/s10113-016-1013-4
Ryschawy, J., Dumont, B., Therond, O., Donnars, C., Hendrickson, J., Benoit, M., et al. (2019). An integrated graphical tool for analysing impacts and services provided by livestock farming. Animal 13, 1760–1772. doi: 10.1017/S1751731119000351
Sancar, F. (2019). Agreement to regulate cell-based meat products. JAMA 321:1449. doi: 10.1001/jama.2019.3831
Schneider, Z. (2012). In vitro meat: space travel, cannibalism, and federal regulation. Houston Law Rev. 50:991. Available online at: https://heinonline.org/HOL/LandingPage?handle=hein.journals/hulr50&div=34&id=&page=
Semba, R. D. (2016). The rise and fall of protein malnutrition in global health. Ann. Nutr. and Metab. 69, 79–88. doi: 10.1159/000449175
Silbergeld, E. K., Graham, J., and Price, L. B. (2008). Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 29, 151–169. doi: 10.1146/annurev.publhealth.29.020907.090904
Simpson, A. (2019). Farmers Wash Up ‘in a Fragile Place' After Historic Midwest Floods. Stateline.Org. Available online at: https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2019/04/25/farmers-wash-up-in-a-fragile-place-after-historic-midwest-floods
Singapore Food Agency (2020). Singapore Food Story RandD Programme. Available online at: https://www.sfa.gov.sg/food-farming/singapore-food-story/r-and-d-programme (accessed May 30, 2020).
Smed, S., Scarborough, P., Rayner, M., and Jensen, J. D. (2016). The effects of the Danish saturated fat tax on food and nutrient intake and modelled health outcomes: an econometric and comparative risk assessment evaluation. Eur. J. Clin. Nutr. 70, 681–686. doi: 10.1038/ejcn.2016.6
Smetana, S., Mathys, A., Knoch, A., and Heinz, V. (2015). Meat alternatives: life cycle assessment of most known meat substitutes. Int. J Life Cycle Assess. 20, 1254–1267. doi: 10.1007/s11367-015-0931-6
Solomon, E. B., Yaron, S., and Matthews, K. R. (2002). Transmission of Escherichia coli O157: H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 68, 397–400. doi: 10.1128/AEM.68.1.397-400.2002
Specht, E. A., Welch, D. R., Clayton, E. M. R., and Lagally, C. D. (2018). Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Biochem. Eng. J. 132, 161–168. doi: 10.1016/j.bej.2018.01.015
Specht, L. (2019). Why Plant-Based Meat Will Ultimately be Less Expensive than Conventional Meat. The Good Food Institute. Available online at: https://www.gfi.org/plant-based-meat-will-be-less-expensive
Springmann, M., Godfray, H. C., Rayner, M., and Scarborough, P. (2016). Analysis and valuation of the health and climate change cobenefits of dietary change. Proc. Natl. Acad. Sci. U.S.A. 113, 4146–4151. doi: 10.1073/pnas.1523119113
Springmann, M., Mason-D'Croz, D., Robinson, S., Wiebe, K., Godfray, H. C. J., et al. (2018). Health-motivated taxes on red and processed meat: a modelling study on optimal tax levels and associated health impacts. PLoS ONE 13:e0204139. doi: 10.1371/journal.pone.0204139
Stephens, N., Di Silvio, L., Dunsford, I., Ellis, M., Glencross, A., and Sexton, A. (2018). Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 78, 155–166. doi: 10.1016/j.tifs.2018.04.010
Stephens, N., Sexton, A. E., and Driessen, C. (2019). Making sense of making meat: key moments in the first 20 years of tissue engineering muscle to make food. Front. Sustain. Food Syst. 3:45. doi: 10.3389/fsufs.2019.00045
Stofferahn, C. W. (2006). Industrialized Farming and its Relationship to Community Well-being: An Update of a 2000. Report by Linda Lobao Department of Sociology, University of North Dakota.
Storm, S. (2017). “Impossible Burger's “Secret Sauce” Highlights Challenges of Food Tech.” The New York Times. Available online at: https://www.nytimes.com/2017/08/08/business/impossible-burger-food-meat.html
Tang, J., Wan, Y., Zhao, M., Zhong, H., Zheng, J. S., and Feng, F. (2020). Legume and soy intake and risk of type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr 111:677–688. doi: 10.1093/ajcn/nqz338
Tasevska, N., Sinha, R., Kipnis, V., Subar, A. F., Leitzmann, M. F., Hollenbeck, A. R., et al. (2009). A prospective study of meat, cooking methods, meat mutagens, heme iron, and lung cancer risks. Am. J. Clin. Nutr. 89, 1884–1894. doi: 10.3945/ajcn.2008.27272
Thorrez, L., and Vandenburgh, H. (2019). Challenges in the quest for ‘clean meat’. Nat. Biotechnol. 37, 215–216. doi: 10.1038/s41587-019-0043-0
Thrupp, L. A. (2000). Linking agricultural biodiversity and food security: the valuable role of agrobiodiversity for sustainable agriculture. Int. Aff. 76, 265–281. doi: 10.1111/1468-2346.00133
Thurstan, R. H., and Roberts, C. M. (2014). The past and future of fish consumption: can supplies meet healthy eating recommendations? Mar. Pollut. Bull. 89, 5–11. doi: 10.1016/j.marpolbul.2014.09.016
Tichenor, N. E., Peters, C. J., Norris, G. A., Thoma, G., and Griffin, T. S. (2017). Life cycle environmental consequences of grass-fed and dairy beef production systems in the Northeastern United States. J. Clean. Prod. 142, 1619–1628. doi: 10.1016/j.jclepro.2016.11.138
Tilman, D., Fargione, J., Wolff, B., D'Antonio, C., Dobson, A., Howarth, R., et al. (2001). Forecasting agriculturally driven global environmental change. Science 292, 281–284. doi: 10.1126/science.1057544
Tömösközi, S., Lásztity, R., Haraszi, R., and Baticz, O. (2001). Isolation and study of the functional properties of pea proteins. Food Nahrung 45, 399–401. doi: 10.1002/1521-3803(20011001)45:6<399::AID-FOOD399>3.0.CO;2-0
Tomova, A., Bukovsky, I., Rembert, E., Yonas, W., Alwarith, J., Barnard, N. D., et al. (2019). The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 6:47. doi: 10.3389/fnut.2019.00047
Tubb, C., and Seba, T. (2019). Rethinking Food and Agriculture 2020-2030: The Second Domestication of Plants and Animals, the Disruption of the Cow, and the Collapse of Industrial Livestock Farming. RethinkX. Available online at: https://www.rethinkx.com/food-and-agriculture
Tuomisto, H. L., Ellis, M., and Haastrup, P. (2014). “Environmental impacts of cultured meat: alternative production scenarios,” in 9th International Conference on Life Cycle Assessment in the Agri-Food Sector (San Francisco, CA). Available online at: https://core.ac.uk/download/pdf/38629617.pdf
Tuomisto, H. L., and Teixeira de Mattos, M. J. (2011). Environmental impacts of cultured meat production. Environ. Sci. Technol. 45, 6117–6123. doi: 10.1021/es200130u
U.S. Department of Agriculture (USDA) (2019). 2017 Census of Agriculture: United States Summary and State Data. Available online at: https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_1_US/
U.S. Department of Agriculture Economic Research Service (USDA ERS). (2019). Recent Trends in GE Adoption. United States Department of Agriculture Economic Research Service (USDA, ERS). Available online at: https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx
Vallaeys, C., Kastel, M., Fantle, W., and Buske, L. (2010). Toxic Chemicals: Banned in Organics but Common in “Natural Food Production: Soy Protein and Chemical Solvents in Nutrition Bars and Meat Alternatives. Cornucopia, WI: The Cornucopia Institute. Available online at: https://www.cornucopia.org/hexane-guides/nvo_hexane_report.pdf
van der Valk, J., Bieback, K., Buta, C., Cochrane, B., Dirks, W. G., Fu, J., et al. (2018). Fetal bovine serum (FBS): past–present–future. ALTEX Altern. Anim. Exp. 35, 99–118. doi: 10.14573/altex.1705101
van der Weele, C., and Driessen, C. (2013). Emerging profiles for cultured meat; ethics through and as design. Animals 3, 647–662. doi: 10.3390/ani3030647
van der Weele, C., and Tramper, J. (2014). Cultured meat: every village its own factory? Trends Biotechnol. 32, 294–296. doi: 10.1016/j.tibtech.2014.04.009
Vijay, V., Pimm, S. L., Jenkins, C. N., and Smith, S. J. (2016). The impacts of oil palm on recent deforestation and biodiversity loss. PLoS ONE 11:e0159668. doi: 10.1371/journal.pone.0159668
Voisin, A.-S., Guéguen, J., Huyghe, C., Jeuffroy, M. H., Magrini, M. B., Meynard, J. M., et al. (2014). Legumes for feed, food, biomaterials and bioenergy in Europe: a review. Agron. Sustain. Dev. 34, 361–380. doi: 10.1007/s13593-013-0189-y
Vyas, D., McGinn, S. M., Duval, S. M., Kindermann, M. K., and Beauchemin, K. A. (2018). Optimal dose of 3-nitrooxypropanol for decreasing enteric methane emissions from beef cattle fed high-forage and high-grain diets. Anim. Prod. Sci. 58, 1049–1055. doi: 10.1071/AN15705
Waters, A. E., Contente-Cuomo, T., Buchhagen, J., Liu, C. M., Watson, L., Pearce, K., et al. (2011). Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 52, 1227–1230. doi: 10.1093/cid/cir181
Weingarten, D. (2018). Why are America's Farmers Killing Themselves? The Guardian, Available online at: https://www.theguardian.com/us-news/2017/dec/06/why-are-americas-farmers-killing-themselves-in-record-numbers
Weinrich, R. (2019). Opportunities for the adoption of health-based sustainable dietary patterns: a review on consumer research of meat substitutes. Sustainability 11:4028. doi: 10.3390/su11154028
Weis, T. (2013). Ecological Hoofprint: The Global Burden of Industrial Livestock. New York, NY: Zed Books.
Welin, S. (2013). Introducing the new meat. Problems and prospects. Etikk i Praksis-Nordic. J. Appl. Ethics. 7, 24–37. doi: 10.5324/eip.v7i1.1788
Wilkinson, J. M. (2011). Re-defining efficiency of feed use by livestock. Animal 5:1014. doi: 10.1017/S175173111100005X
Willett, W., Rockström, J., Loken, B., Springmann, M., Lang, T., Vermeulen, S., et al. (2019). Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393, 447–492. doi: 10.1016/S0140-6736(18)31788-4
Williams, G. M., Berry, C., Burns, M., de Camargo, J. L. V., and Greim, H. (2016). Glyphosate rodent carcinogenicity bioassay expert panel review. Crit. Rev. Toxicol. 46(Suppl. 1), 44–55. doi: 10.1080/10408444.2016.1214679
Williams, M., Stout, J., Roth, B., Cass, S., Pappa, V., and Rees, B. (2014). Legume Futures Report 3.7: Environmental Implications of Legume Cropping. Legume Futures. Available online at: http://www.legumefutures.de/images/Legume__Futures__Report__3.7.pdf
Wing, S., Horton, R. A., and Rose, K. M. (2013). Air pollution from industrial swine operations and blood pressure of neighboring residents. Environ. Health Perspect. 121, 92–96. doi: 10.1289/ehp.1205109
Zhang, G., Zhao, X., Li, X., Du, G., Zhou, J., and Chen, J. (2020). Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 97, 443–450. doi: 10.1016/j.tifs.2020.01.026
Zhang, X., Shu, X. O., Li, H., Yang, G., Li, Q., Gao, Y. T., et al. (2005). Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch. Intern. Med. 165, 1890–1895. doi: 10.1001/archinte.165.16.1890
Zheng, Y., Li, Y., Satija, A., Pan, A., Sotos-Prieto, M., Rimm, E., et al. (2019). Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ 365:l2110. doi: 10.1136/bmj.l2110
Zhu, X., and van Ierland, E. C. (2004). Protein chains and environmental pressures: a comparison of pork and novel protein foods. Environ. Sci. 1, 254–276. doi: 10.1080/15693430412331291652
Keywords: meat alternative, meat substitute, meat analog, cellular meat, seafood alternative, greenhouse gas emissions, land use, water footprint
Citation: Santo RE, Kim BF, Goldman SE, Dutkiewicz J, Biehl EMB, Bloem MW, Neff RA and Nachman KE (2020) Considering Plant-Based Meat Substitutes and Cell-Based Meats: A Public Health and Food Systems Perspective. Front. Sustain. Food Syst. 4:134. doi: 10.3389/fsufs.2020.00134
Received: 03 June 2020; Accepted: 27 July 2020;
Published: 31 August 2020.
Edited by:
Cristobal N. Aguilar, Universidad Autónoma de Coahuila, MexicoReviewed by:
Jean-Francois Hocquette, INRA UMR1213 Herbivores, FranceRomeo Rojas, Autonomous University of Nuevo León, Mexico
Copyright © 2020 Santo, Kim, Goldman, Dutkiewicz, Biehl, Bloem, Neff and Nachman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keeve E. Nachman, knachman@jhu.edu
 Raychel E. Santo
Raychel E. Santo Brent F. Kim
Brent F. Kim Sarah E. Goldman
Sarah E. Goldman Jan Dutkiewicz
Jan Dutkiewicz Erin M. B. Biehl
Erin M. B. Biehl Martin W. Bloem
Martin W. Bloem Roni A. Neff
Roni A. Neff Keeve E. Nachman
Keeve E. Nachman